15 GI
Acute Mesenteric Ischemia
Acute mesenteric ischemia (AMI) is a syndrome in which there is acute onset of inadequate blood flow through the mesenteric vessels, which commonly results from occlusion or hypoperfusion to the vessels.
Common causes of acute mesenteric ischemia include:
Arterial thrombus due to atherosclerosis (most common)
Venous thrombus due to hypercoagulable states (polycythemia vera, pregnancy, protein C and S deficiency, etc)
Arterial occlusion due to emboli from an arrhythmia or post-cardiac catheterization
Hypoperfusion due to blood loss or CHF
Chronic mesenteric ischemia (CMI) is ischemia of the bowel due to long-standing atherosclerotic disease of 2 or more mesenteric vessels. Vasculitides may also cause chronic mesenteric ischemia.
Chronic mesenteric ischemia is a rare diagnosis, usually affecting people over the age of 60, and is more common in females than males.
The three vessels that may be affected in chronic mesenteric ischemia are the celiac trunk, superior mesenteric artery, and inferior mesenteric artery. Due to collateral flow, 2 of 3 must be affected to cause clinically significant disease.

Symptoms
In patients with acute mesenteric ischemia, symptoms may include:
Non-localized, diffuse, and constant abdominal pain
Nausea
Vomiting
Bloody diarrhea
In chronic mesenteric ischemia, patients present with:
Postprandial pain (epigastric or periumbilical)
Fear of eating (sitophobia)
Weight loss
History of vascular disease
Nausea, vomiting, diarrhea
On physical exam, patients with acute mesenteric ischemia may have the following:
Pain disproportionate to physical exam findings
Abdominal distention
Fever
Hypotension
Tachycardia
Altered mental status
Positive fecal occult blood
Patients with chronic mesenteric ischemia may have the following:
Pain disproportionate to physical exam findings
Abdominal bruit
Decreased peripheral pulses
Signs of weight loss
Diagnosis
Abdominal CT may be helpful by showing focal or segmental bowel wall thickening. The gold standard for diagnosis of acute or chronic mesenteric ischemia is CT angiography.
In acute and chronic mesenteric ischemia, other helpful imaging modalities may include abdominal x-ray and magnetic resonance angiography.
In both acute and chronic mesenteric ischemia, laboratory studies are typically non-specific, but may include:
Elevated WBC count with left shift
Elevated hematocrit due to hemoconcentration
Increased lactate dehydrogenase
Elevated amylase, compared to lipase for pancreatitis
Metabolic acidosis
Prothrombin time, activated partial thromboplastin time, and international normalized ratio abnormalities
In addition to those above, in chronic mesenteric ischemia, the following may be found:
Anemia, leukopenia, lymphopenia due to chronic malnourishment
Hypoalbuminemia due to malnourishment
Electrolyte abnormalities due to vomiting, diarrhea, or malnutrition
Plain films of the abdomen are typically normal in acute mesenteric ischemia, but are sometimes warranted to exclude a perforated viscus and free air under the diaphragm.
CT scan: Colonic wall thickening, fat stranding
Endoscopy: Edematous & friable mucosa
If colon resection is not needed, IV abx and colonoscopy is used to confirm diagnosis.
Complications
Acute mesenteric ischemia may result in:
Bowel necrosis
Perforation
Peritonitis
Sepsis
Death
Complications of chronic mesenteric ischemia include acute thrombosis or embolism, and prolonged hospitalization due to chronic malnutrition.
Treatment
The initial goal in treating acute mesenteric ischemia includes fluid resuscitation, placement of nasogastric tube, and IV antibiotics. Once stabilized, patients will undergo embolectomy (ie clot removal) plus thrombolytic infusion via the angiography catheter.
Necrotic bowel appears dusky and blue on gross pathologic examination. When bowel necrosis is present, patients require a laparotomy in order to resect the necrotic bowel.
Chronic mesenteric ischemia is treated with open or endovascular revascularization. Anticoagulation with warfarin is also indicated.
Appendicitis
Appendicitis is inflammation of the appendix.
Common causes of appendicitis include:
Lymphoid tissue hyperplasia (60%) is the most common overall and is a more common cause in younger patients.
Fecaliths (30%) are a common cause in elderly patients.
Foreign bodies
Infection (eg. parasites)
Neoplasia (eg. carcinoid tumor, adenocarcinoma)
In children, acute appendicitis is the most common disease that requires emergency surgery and is also one of the most frequently diagnosed.
Symptoms
The classic presentation of appendicitis is characterized by abdominal pain that typically originates in the periumbilical region and shifts towards the RLQ followed by nausea, vomiting and anorexia.
The RLQ pain experienced by patients with appendicitis most often occurs at McBurney’s point, 2/3 the distance from the umbilicus to the right anterior superior iliac spine.
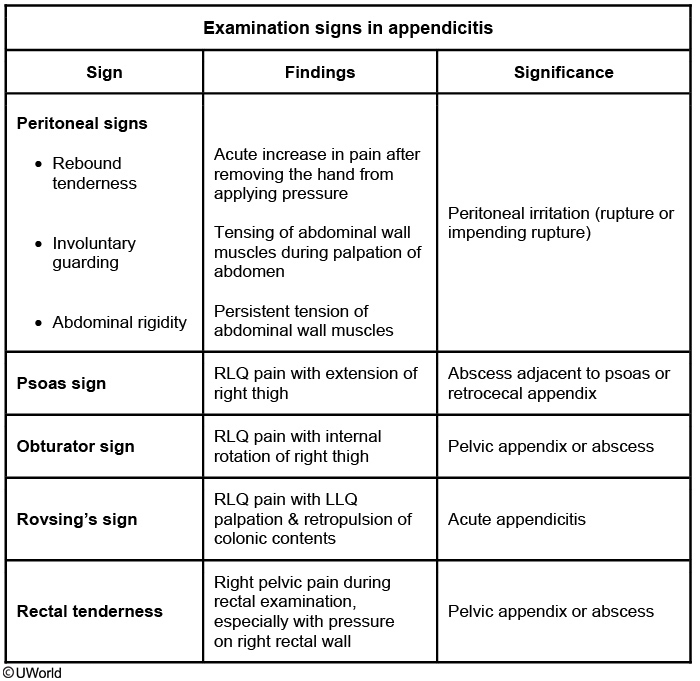
In addition to rebound tenderness and RLQ pain, three classic signs are often seen in patients with appendicitis including:
Rovsing’s sign - referred pain to the RLQ upon palpation of the LLQ.
Psoas sign - RLQ pain with right hip flexion or extension.
Obturator sign - RLQ pain elicited with right thigh flexion and internal rotation while in the supine position.
Diagnosis
Diagnosis is based on a thorough clinical assessment as imaging studies and laboratory findings can only support a diagnosis of acute appendicitis.
Ultrasound is the diagnostic test of choice in pregnant woman and young children. Findings seen on an ultrasound include a "target appearance", dilated lumen and periappendiceal fluid collection.
CT scan is the diagnostic test of choice in patients with suspected appendicitis as it is the most sensitive and specific imaging modality. Imaging will reveal thickened enhancing walls, periappendiceal inflammation including fat stranding and a dilated appendiceal lumen ( > 6mm diameter).
Patients with appendicitis may have a mild to moderate leukocytosis with a left shift (WBC > 10,000).
Treatment
The definitive treatment of acute appendicitis is appendectomy. Medical management with antibiotics alone is NOT the standard of care in patients who present with clinical and radiographic findings consistent with appendicitis.
In the pediatric population, appendectomy on the day of diagnosis is the treatment of choice for unruptured appendicitis.
Supportive measures for patients with suspected appendicitis include:
IV fluids
Broad-spectrum antibiotics
Electrolyte monitoring
There are several complications that can occur in a patient with appendicitis including gangrenous perforation and abscess formation.
Appendiceal abscess
Patients who have a delayed presentation with a longer duration of symptoms (>5 days, as in this patient) often have appendiceal rupture with a contained abscess. These patients will generally have significant fever and leukocytosis, but findings on anterior palpation of the abdomen may be unrevealing. In such cases, maneuvers that assess the deep abdominal spaces (eg, psoas sign, obturator sign, rectal examination) may be more informative. In particular, the psoas sign suggests the presence of an abscess posterior to the appendix adjacent to the psoas muscle (or possibly an unruptured retrocecal appendix). Computed tomography imaging can confirm the diagnosis in these cases. Patients with a contained appendiceal abscess have a very high complication rate from immediate surgery due to the mass of inflamed, infected, and friable debris and adhesions. If they are otherwise clinically stable, these patients should be managed with intravenous antibiotics, bowel rest, and possibly percutaneous drainage of the abscess. They can return in 6-8 weeks for appendectomy on an elective basis ("interval appendectomy").

This patient with a recent history of skin infection who presents with fever and abdominal pain radiating to the groin likely has a psoas abscess (PA). PA occurs from either hematologic seeding from a distant infection or from direct extension of an intraabdominal infection (eg, diverticulitis, vertebral osteomyelitis). Risk factors include HIV, intravenous drug use, diabetes, and Crohn disease.
PA frequently presents subacutely with fever and lower abdominal or flank pain, although symptoms (eg, anorexia, weight loss) can be nonspecific. Consequently, PA should be considered as part of the evaluation for fever of unknown origin. Deep abdominal palpation is required to elicit tenderness due to the location of the psoas on the posterior abdominal wall. The "psoas sign," abdominal pain with hip extension, can often be detected on examination. Laboratory studies commonly show leukocytosis, thrombocytosis, and elevated inflammatory markers. CT scans are required to confirm the diagnosis. Drainage is critical, and blood and abscess cultures should be obtained to guide antibiotic therapy.

Colorectal cancer diagnosis, treatment
Clinically, colonoscopy with biopsy is the gold standard for diagnosis of colorectal adenocarcinomas.
Histologically on biopsy, colorectal adenocarcinomas consist of dysplastic columnar cells with some varying degree of epithelial differentiation.
Biopsy showing mucinous differentiation and signet-ring formation is more commonly seen on the right side and carries a poorer prognosis.
By far, the most important prognostic factors are:
Tumor invasion
Lymph node involvement
Metastasis
This is the classic TNM tumor staging.
T0 = Carcinoma confined to the mucosa or carcinoma in situ
T1 = Invasion of the submucosa
T2 = Invasion of the muscularis propria
T3 = Invasion of the serosa
T4 = Invasion of other organs or the peritoneum
The Duke Classification System can be used for determining prognosis based on a given tumor stage: Class
Equivalent TNM Stage
Description
Cure Rate
A
I
Tumor confined to bowel wall
90%
B
II
Penetration of tumor into colonic serosa or perirectal fat
80%
C
III
Lymph node involvement
<60%
D
IV
Distant metastases
<5%
Patients who have completed active treatment for colorectal cancer and have no evidence of disease should undergo the following surveillance:
History and physical exam and carcinoembryonic antigen (CEA) testing every 3-6 months for the first 5 years
Abdomen and chest CT scan annually for at least 3 years
Colonoscopy at 1 year
Note: Specific recommendations differ slightly between organizations ASCO/CCO and NCCN. The above are recommendations common to both.
Additional lab findings that may assist in the diagnosis of colorectal cancer include a complete blood count showing decreased hemoglobin and hematocrit, and a positive stool guaiac test.
Treatment prioritizes resection if the cancer is localized. Adjuvant chemotherapy may be appropriate if lymph nodes are involved.
Prevention of colorectal cancer consists of cancer screening and surveillance. The timing and frequency of these depend on the patient's age and risk factors.
Average risk patients (no family history and no signs/symptoms): Beginning at age 50 and continuing to age 75, USPSTF recommends one of the following:
Fecal occult blood test (FOBT) every year
FOBT every 3 years + flexible sigmoidoscopy every 5 years
Colonoscopy every 10 years
Note: colonoscopy is more sensitive than sigmoidoscopy, and is considered the preferred screening method.
Screening colonoscopy in patients with a first-degree relative that has been diagnosed with colorectal cancer <60 years of age should be initiated either:
At age 40
OR
10 years younger than the age at which the relative was diagnosed
Whichever of the above ages comes first should be used to initiate screening colonoscopy. It should be repeated every five years in these patients.
Colorectal Cancers Causes
Hereditary causes of colorectal cancer include:
Familial adenomatous polyposis (FAP)
Hereditary non-polyposis colorectal cancer (HNPCC)
Gardner syndrome
Peutz-Jeghers syndrome
Turcot syndrome
Juvenile polyposis
Familial adenomatous polyposis consists of hundreds of polyps in the colon. Development of colorectal cancer is near-certain, so prophylactic subtotal colectomy is typically recommended in these patients.
Hereditary non-polyposis colorectal cancer arises from multiple genetic mutations. Neoplasms usually form in the proximal colon from normal-appearing mucosa.
Gardner syndrome is similar to familial adenomatous polyposis, with large amounts of polyps in the colon. Bone and soft tissue tumors (osteomas of the mandible and skull, epidermal cysts, desmoid tumors) are also seen.
Hallmarks of Peutz-Jeghers syndrome include hamartomatous polyps with low malignant risk, and mucocutaneous pigmentation of the mouth, hands, and genitals.
In Turcot syndrome, an autosomal recessive disorder, colonic adenomas with high malignant potential are seen. These frequently become malignant in those below the age of 30. Malignant CNS tumors such as glioblastoma multiforme and medulloblastoma are also seen.
Juvenile polyposis consists of polyps in the colon, small bowel, and stomach. It is associated with painless rectal bleeding, rectal prolapse, and failure to thrive. Risk of malignancy is slightly increased later in life.
Mutations in the adenomatous polyposis coli (APC) gene are implicated in the development of:
Familial adenomatous polyposis
Gardner syndrome
Turcot syndrome
Those who have a first-degree relative with FAP should undergo yearly screening with flexible sigmoidoscopy or colonoscopy beginning at age 10-12. Yearly screening can be stopped after age 40 if no adenomas are detected.
Crohn's
Crohn’s Disease (CD) is an idiopathic inflammatory disorder that can affect any portion of the gastrointestinal (GI) tract, resulting in relapsing and remitting episodes of abdominal pain and diarrhea.
Crohn's disease is thought to be due to a dysregulated proinflammatory response to the bacteria lining the walls of the GI tract, which ultimately results in the release of inflammatory substances that cause direct mucosal injury.
There is a bimodal distribution with the first peak occurring between ages 15-30, then a second peak between 60-70 years of age. It is also more common in caucasians.
Patients with CD typically present with “crampy” abdominal pain, diarrhea, fatigue, weight loss, and growth failure.
Specific to children, growth failure is a particularly important presenting feature of Crohn’s disease; growth failure specifically refers to a vertical growth rate below the appropriate velocity for age.
In children, Crohn’s disease most commonly involves the ileum and colon (40-50%), followed by the terminal ileum only, followed by the colon only.
When CD affects the colon, it can be very difficult to distinguish from ulcerative colitis.
CD can also present with the same extraintestinal manifestations seen with ulcerative colitis, such as:
Ankylosing spondylitis (arthritis of the spine)
Uveitis
Pyoderma gangrenosum
Erythema nodosum
Calcium oxalate kidney stones due to increased absorption of oxalate
Gallstones with terminal ileum involvement due to decreased absorption of bile acids
Diagnosis
The best initial diagnostic tool used in CD is endoscopy with mucosal biopsy of the small intestine and colon, which will reveal skipped areas of inflammation, cobblestoning of the mucosa, strictures, and even pseudopolyps. Biopsy will also reveal transmural inflammation and granulomas, which are not seen with ulcerative colitis.
Various imaging studies are also used in the diagnosis of CD, such as: barium enema and small bowel follow-through (patient swallows contrast while images are taken through the GI tract).
The major complications seen with CD are intestinal perforation, strictures (narrowing of lumen that leads to bowel obstruction), fistulas, sinus tracts, and the development of cancer in areas of inflammation such as the colon (if involved).
Treatment
Oral 5-aminosalicylic acid (5-ASA) drugs (e.g. sulfasalazine, mesalamine) are used to treat mild to moderate Crohn's disease.
Severe Crohn's disease is treated with steroids and antimetabolites (e.g. azathioprine, 6-mercaptopurine) and methotrexate.
Antibiotics (e.g. ciprofloxacin, metronidazole) can be used in patients with mild to moderate Crohn's disease that do not respond to 5-aminosalicylic acid therapies.
Biologic therapies are becoming more widely used. Infliximab (Remicade) and Adalimumab (Humira) are monoclonal antibodies against TNF-alpha, which is involved in systemic inflammation and stimulation of the acute phase reaction.
These may be incorporated into the treatment regimen of a moderate to high risk patient, as defined by the following guidelines according to the American Gastroenterological Association:
Age at initial diagnosis < 30 years
Extensive anatomic involvement
Perianal and/or severe rectal disease
Deep ulcers
Prior surgical resection
Stricturing and/or penetrating behavior
Surgical intervention is recommended when any of the following complications are present:
Obstruction
Abscess
Fistula or stricture formation
Bowel perforation
Toxic megacolon
Cancer
Ulcerative Colitis
Ulcerative colitis is a type of inflammatory bowel disease that disrupts the mucosal lining of the rectum and colon, resulting in recurrent episodes of abdominal pain and bloody diarrhea.
The exact etiology of ulcerative colitis is still unknown, but it has been linked to:
Genetic factors (i.e. a family history of ulcerative colitis)
Immune system reactions (most frequently the presence of p-ANCA antibodies)
Environmental factors
NSAID use
Note: Smoking is a protective factor.
Ulcerative colitis is slightly more common in men and occurs more frequently in Caucasians when compared to other races. There is also a bimodal age distribution that peaks between 15-25 years of age, then again between 55-65 years of age.
Clinical features of ulcerative colitis include:
Hematochezia (bloody diarrhea)
Abdominal pain
Frequent, small volume bowel movements
Rectal mucus discharge
Tenesmus (feeling of incomplete evacuation of stools, despite an empty colon)
Various extraintestinal symptoms have also been associated with ulcerative colitis, such as:
Uveitis (inflammation of the middle layer of the eye)
Pyoderma gangrenosum (necrotic ulcerations of the legs)
Ankylosing spondylitis (chronic inflammation of the spine)
Primary sclerosing cholangitis (sclerosis of both intrahepatic and extrahepatic bile ducts)
The diagnostic modality of choice for ulcerative colitis is colonoscopy with mucosal biopsy.
Colonoscopy will typically show a friable erythematous mucosa, with or without ulcerations, and pseudopolyps, that extends continuously from the rectum to part of or the entire colon.
Biopsy typically reveals inflammation limited to the mucosa, submucosa,and crypt abscesses. The inflammation is not transmural.
Laboratory studies will typically reveal microcytic anemia secondary to iron deficiency, and elevated inflammatory markers (erythrocyte sedimentation rate (ESR) and elevated C-reactive protein).
Imaging is typically not required for diagnosing ulcerative colitis, but plain upright x-rays can help diagnose toxic megacolon or colonic perforation in the acute setting.
Double-contrast barium enema can be used if ulcerative colitis is suspected,but should be avoided during an acute attack due to the risk of perforation. Characteristic findings on barium enema include:
Microulcerations
Pseudopolyps
Narrowing of luminal wall
Loss of haustra ("lead pipe" appearance)
Complications of ulcerative colitis include:
Increased risk of colorectal cancer
The risk begins 8-10 years after the initial diagnosis of pancolitis, and 12-15 years after the onset of left-sided colitis. At this point in time, colonoscopies every 1-2 years with biopsies for dysplasia are recommended.
Severe bleeding
Strictures
Colonic perforation
Toxic megacolon
Patients with ulcerative colitis are at greatest risk of developing toxic megacolon early in the course of the disease (within 3 years of diagnosis).
Plain abdominal radiographs in patients with toxic megacolon classically reveal thick haustral markings that do not extend across the entire lumen.

Vital signs and labs indicative of ulcerative colitis include:
Fever >38ºC
Heart rate >120 beats/min
Neutrophilic leukocytosis >10,500/µl
Anemia
Hypotension
Initial mainstay treatment of mild ulcerative colitis includes topical (suppository, enema) and oral 5-aminosalicylic acid (5-ASA) medications (mesalazine, sulfasalazine).
High dose corticosteroids are used during acute, severe ulcerative colitis attack.
Medical management of patients with toxic megacolon and ulcerative colitis includes:
Complete bowel rest
Correction of laboratory abnormalities
Broad-spectrum antibiotics (e.g. ampicillin-gentamicin-metronidazole)
IV corticosteroids: first line treatment
Bowel decompression (via nasogastric or long intestinal tube)
Surgery is reserved for patients who:
Fail medical treatment
Perforate
Contract colon cancer
Have severe toxic megacolon
Gastroparesis
Gastroparesis is a syndrome in which there is delayed gastric emptying, due primarily to autonomic dysfunction, NOT mechanical obstruction.
Pre-disposing factors include:
Diabetes mellitus
Post-surgical procedures (e.g. gastrectomy, Nissen fundoplication)
Medication use (e.g. narcotics, calcium channel blockers, and tricyclic antidepressants)
Symptoms
Patients typically present with symptoms of:
Nausea
Vomiting
Abdominal pain
Bloating
Early satiety
On physical exam patients may present with:
Distended abdomen
Abdominal tenderness
Succussion splash (an ausculatory sound that the stomach contents make when they hit the pylorus)
Diagnosis
The best test in diagnosing gastroparesis is a scintigraphic gastric emptying study.
For a gastric emptying test, a patient ingests a low fat egg white meal and gastric emptying is measured at 1, 2, and 4 hours. Greater than 10% retention is a positive test.
Before a gastric emptying study is performed, a mechanical obstruction (e.g. mass, hiatal hernia) should be ruled out using a CT, MRI, or upper endoscopy.
Treatment, Complications
Treatment consists of:
Dietary modifications (i.e. blenderized food)
Medications
Surgery in refractory cases
Pro-kinetic medications help improve gastric emptying time. They include:
Metoclopramide
Domperidone
Erythromycin
For patients who are refractory to pharmacological therapy, a percutaneous gastrostomy tube may be placed to decompress and drain the stomach. Other invasive options include implantation of a gastric pacemaker and intrapyloric botox injections.
Gastroparesis may result in long term complications such as:
Weight loss
Malabsorption
Tooth decay
Iron deficiency anemia
Infection
Diverticulitis
Diverticulitis is the acute inflammation of a diverticulum (mucosal outpouching of the bowel wall) of diverticulosis. This may be due to obstruction (fecal matter, undigested food particles), vascular compromise and perforation, or increased intraluminal pressure leading to erosion of the diverticular wall.
Diverticulitis is a complication of diverticulosis, but not all patients with diverticulosis will develop diverticulitis (about 15-20%).
Diet and lifestyle modifications, such as increased dietary fiber, stool softeners, or fluids for diverticulosis decrease risk for diverticulitis.
Patients with diverticulitis classically present with the triad of:
Left lower quadrant abdominal pain
Fever
Leukocytosis
Additional symptoms that patients may have are:
Nausea
Vomiting
Constipation
Diarrhea
A painful rectal mass may be palpable if foci of inflammation are near the rectum.
Diagnosis
CT scan of the abdomen and pelvis with oral and IV contrast is definitive for diagnosis or exclusion of diverticulitis. CT scan can show the inflammatory changes as soft-tissue stranding and colonic wall thickening.
Colonoscopy and barium studies are contraindicated in patients with diverticulitis due to the high risk of bowel wall perforation.
Contrast this with the diverticulosis, in which barium enema is the definitive diagnostic test.
Abdominal x-rays are useful for excluding other diagnoses, such as ileus or obstruction.
Complications
Complications of diverticulitis occur when persistent inflammation results in the following:
Abscess formation (most common)
Colonic obstruction
Colon perforation
Colovesical fistula
Colovesical fistula is an abnormal connection between the colon and bladder that can be caused by diverticulitis. Manifestations of colovesical fistula include:
Pneumaturia (air in urine)
Fecaluria (feces in urine)
Recurrent polymicrobial UTIs
Pneumaturia and fecaluria are virtually pathognomonic for colovesical fistula.
The diagnosis of colovesical fistula is established with a CT scan of the abdomen and pelvis with either oral or rectal (NOT intravenous) contrast.
In patients with colovesical fistula, this will show contrast in the bladder and localized thickening of the bladder and colon walls.
After diagnosis of a colovesical fistula is confirmed by CT, patients should undergo colonoscopy to rule out a malignancy of the colon.
Abscesses measuring <3cm (ring enhancing fluid collection) are not amenable to be treated by CT-guided percutaneous drainage and may resolve with antibiotics alone; those measuring >3cm should undergo CT-guided percutaneous drainage.
If the abscess cavity contains gross fecal material or if perforation has occurred, surgery is required.
Colovesical fistulas can be treated with surgery.
Young patients will have a greater total amount of recurrent infections because the diverticular disease does not disappear with age.
Treatment
Patients with uncomplicated diverticulitis should receive the following initial treatment:
Made nil per os (NPO)
Broad-spectrum antibiotics for 7-10 days
IV fluids
Pain control
Mild, uncomplicated diverticulitis may be managed outpatient if the patient has no comorbidities.
Antibiotic therapy in diverticulitis should cover E. coli and anaerobes. Some pharmacologic options are:
Metronidazole + ciprofloxacin
Amoxicillin/clavulanate
Ticarcillin/clavulanate
Piperacillin/tazobactam
Ertapenem
Surgical therapy is pursued in the following situations:
No response to antibiotics
Frequent recurrent infections
Complications (perforation, fistula formation, abscess, stricture, obstruction)
Complicated diverticulitis refers to diverticulitis associated with an abscess (as seen in this patient), perforation, obstruction, or fistula formation. A fluid collection less than 3 cm can be treated with intravenous antibiotics and observation, with surgery reserved for patients with worsening symptoms. However, a fluid collection >3 cm should have CT-guided percutaneous drainage. If the symptoms are not controlled by the fifth day, surgical drainage and debridement are recommended. Sigmoid resection is generally reserved for patients with fistulas, perforation with peritonitis, obstruction, or recurrent attacks of diverticulitis.
Diverticulosis
Diverticulosis is the presence of multiple (often dozens) colonic diverticula (mucosal outpouchings). It is the most common cause of overt lower gastrointestinal bleeding (hematochezia) in adults.
Diverticula occur where the vasa recta enters muscularis externa, which are areas of intrinsic weakness. These are false diverticula, as they don't involve all bowel wall layers.
Diverticula formation is due to chronically increased intraluminal pressure from low-fiber diet (Western diet), hence the age-dependent prevalence.
Diverticulosis most commonly occurs in the sigmoid colon.
Patients are typically asymptomatic and diverticula may be detected as incidental findings on screening colonoscopy, and may also be seen with barium enema.
Bleeding associated with diverticulosis most commonly presents as painless hematochezia.
The bleeding associated with diverticulosis resolves spontaneously without intervention in most patients.
Once an upper gastrointestinal source of bleeding has been ruled out by upper endoscopy or nasogastric lavage, colonoscopy is performed to localize the site of diverticular bleeding.
Patients with diverticulosis are recommended to increase their consumption of fiber.
Dysphagia
Dysphagia is difficulty with swallowing.
Dysphagia can be the result of:
Neuromuscular pathologies (myasthenia gravis, scleroderma, achalasia)
Obstruction (tumors, esophageal webs)
Dysfunction in esophageal transport
Painful swallowing (odynophagia)
Neuromuscular dysfunction interferes with swallowing solids and liquids.
Patients with dysphagia complain of feeling like food is stuck in their throat and coughing when trying to swallow.
A barium swallow and EGD can help identify the underlying cause of dysphagia. Manometry is useful in identifying neuromuscular etiologies.
General treatment options include:
Dietary modification
Training in swallowing techniques
Pharmacologic treatment
GI Bleed
Patients with gastrointestinal bleeding may present with a constellation of abnormal vital signs along with:
Hematemesis
Hematochezia
Melena
Anemia
Hypotension
Pallor
Abdominal pain
Fatigue
In an acute GI bleed, there can be marked hemodynamic instability. Early clinical signs include hypotension, tachycardia and orthostatic symptoms.
Rapid GI bleeding can present with hypovolemic and hemorrhagic shock.
In an acute, briskly moving GI bleed, a normocytic, normochromic anemia can be seen (loss of both red cell mass and plasma volume).
One-third of cases of presumed lower GI bleeding are actually caused by very brisk upper GI bleeding.
Slow, chronic bleeding can present as an iron deficiency anemia (e.g. colonic malignancy)
PUD and gastritis can present with abdominal pain in addition to melena.
GI bleeding can be divided into upper and lower GI tracts (separated by the Ligament of Treitz at the duodenum-to-jejunum junction), both evaluated and managed with similar yet distinct algorithms.
Upper GI bleeding typically presents with “coffee-ground” emesis, hematemesis, and melena. Lower GI Bleeding is more likely to present with hematochezia.
Upper GI bleeding is most likely due to:
Peptic ulcer disease
Gastritis
Esophageal varices
Lower GI bleeding is caused by:
Diverticulosis
Inflammatory bowel disease (IBD)
Angiodysplasia (AVM)
Hemorrhoids
Colonic malignancy
Initial assessment of gastrointestinal bleeding includes:
ABCs (Airway, Breathing, Circulation)
Complete blood count
Fecal occult blood test
Rectal exam
Hemodynamic monitoring
Nasogastric tube placement (for both upper and lower GI bleeding)
For upper GI bleeding, endoscopy is used for visualization once the patient is hemodynamically stable and a nasogastric tube has been placed.
For hematochezia in hemodynamically stable patients, colonoscopy or sigmoidoscopy may be used following NG tube placement to evaluate for lower GI bleeding.
In hemodynamically unstable patients, an upper GI source should be considered, and an upper endoscopy should be performed once the patient is stabilized.
For active GI bleeding, options for diagnosis may include:
Bleeding scan: Low in sensitivity for detecting GI bleed.
Mesenteric angiography: May not be diagnostic if the bleeding has already stopped.
For a patient who is hemodynamically unstable and having an active bleed, angiography may be the best initial study to help localize the area of bleeding.
Other pertinent labs may include:
Coagulation studies (PT, aPTT and INR can be used to document the presence of a coagulopathy, and can help assess continued bleeding)
Liver function tests (levels can be indicative of hepatocellular injury, which may be responsible for poor synthetic function and continued bleeding)
BUN/Cr (blood in the upper intestine can raise BUN, and an elevated BUN/Cr ratio increases with upper GI bleeding)
Initial management of a gastrointestinal bleed includes minimizing blood loss, identifying and stopping the source of bleeding, and maintaining hemodynamic stability. Long-term management is directed at the underlying etiology.
Monitoring for hemodynamic stability, establishment of large bore IV access, and emergent and aggressive volume resuscitation, if clinically indicated, are used to maintain blood pressure and tissue perfusion. The initial fluid of choice for volume resuscitation is isotonic (i.e. normal) saline.
The correction of any underlying coagulopathy is essential. For someone on Warfarin (Coumadin), fresh frozen plasma (FFP) should be used to correct the coagulopathy emergently.
An intravenous Proton Pump Inhibitor (PPI) can be used for gastric acid suppression for upper GI bleeds. PPIs are helpful because the acidity of the stomach inactivates the coagulation factors.
Intravenous somatostatin (Octreotide) is used in the management of esophageal varices.
Following a type and crossmatch of the patient, 1 unit of packed red blood cells should replenish hemoglobin by 1 unit and hematocrit by 3 units. Serial Hb/Hct should be obtained to monitor for ongoing bleeding.
Hematemesis

This patient has hematemesis in the setting of a history of peptic ulcer disease and alcoholic cirrhosis, which places him at risk for rapid, life-threatening upper gastrointestinal bleeding (UGIB). Therefore, prompt and aggressive fluid resuscitation should be the first step in management. All patients with acute hemorrhage should have vascular access established immediately with at least 2 large-bore intravenous lines (2, not just 1).
Once appropriate vascular access has been established, fluid resuscitation with crystalloid should be undertaken while any necessary blood products are prepared for transfusion (eg, packed red blood cell transfusion to maintain hemoglobin at least >7 g/dL). In addition, patients' airway, vital signs, cardiac rhythm, and urine output should be closely monitored. Patients with UGIB should also receive acid suppression with intravenous proton pump inhibitors and have all oral intake restricted. If variceal bleeding is suspected, as in this case, an octreotide infusion should be considered (Choice A). Patients with cirrhosis should also receive prophylactic antibiotics.
Once patients are stabilized, upper endoscopy should be performed to identify and control the source of hemorrhage and to prevent recurrent episodes (Choice C). In approximately 50% of cases of variceal bleeding, the hemorrhage ceases on its own without further intervention; this rate is significantly lower than that seen in UGIB due to other causes, which approaches 90%.
Abnormal LFT
Alanine Aminotransferase (ALT) and Aspartate Aminotransferase (AST) are used as markers of hepatic damage.
When aminotransferase levels are mildly elevated, on the order of several hundred, suspect:
Chronic hepatitis
Alcohol-induced hepatitis
Nonalcoholic fatty liver disease
When aminotransferases levels are moderately elevated, on the order of several hundred to several thousand, suspect acute viral hepatitis.
When the aminotransferases levels are severely elevated, generally greater than 10,000, suspect significant liver damage caused by:
Toxin-related hepatitis (eg. acetaminophen overdose)
Liver ischemia
Elevations in ALT are MOST specific to liver pathology since AST can be found in skeletal and cardiac muscle in addition to the liver.
Elevations of AST and ALT usually increase in a ratio that is roughly 1:1. One notable exception is alcoholic liver disease where the ratio of AST to ALT is usually greater than 2:1.
The cholestatic pattern of liver disease shows:
Markedly elevated alkaline phosphatase & GGT
Only mildly elevated ALT & AST
The hepatic pattern of liver disease shows
Markedly elevated AST & ALT
Minimal to no elevation in alkaline phosphatase
Alkaline phosphatase is elevated when there is obstruction in the biliary tree. It suggests cholestasis, but is not specific to the liver.
Serum levels of gamma-glutamyl transpeptidase (GGT) can be especially useful in determining the etiology (liver or bone) of an elevated alkaline phosphatase since this enzyme is not present in bone. If both the alkaline phosphatase and GGT are elevated, the pathology is likely of hepatic origin. If GGT is normal, however, the elevated alkaline phosphatase may be due to pregnancy (placenta produces alkaline phosphatase) or pathology related to bone (eg. Paget disease, osteomalacia, osteosarcoma).
Low levels of serum albumin are consistent with patients who have chronic liver disease such as advanced liver cirrhosis. In patients with acute liver failure or significant liver injury, albumin levels are often NORMAL in the short term since the half-life of albumin is 20 days.
Severe liver damage can be reflected by changes in prothrombin time and INR since vitamin-K dependent clotting factors II, VII, IX, and X are synthesized in the liver.
Ascites
Ascites is a fluid collection within the peritoneum usually found in the setting of:
Portal hypertension causing increased hydrostatic pressure (e.g. cirrhosis, right sided heart failure, Budd-Chiari Syndrome)
Hypoalbuminemia causing decreased oncotic pressure (e.g. malnutrition, nephrotic syndromes)
Malignancies (e.g. ovarian cancer)
Infections (e.g. tuberculosis)
Patients with ascites generally present with:
Abdominal swelling
Weight gain
Shortness of breath
Associated symptoms from the etiology of the ascites (e.g. hepatic stigmata)
The diagnosis of ascites is usually based on history, physical exam, ultrasound, and paracentesis.
Physical exam findings on patients with ascites include:
Bulging flanks
Fluid waves
Shifting dullness on percussion (changing location of dullness when the patient turns)
Associated findings related to ascites etiology
Paracentesis
A paracentesis can be performed to help determine the etiology of the ascites.
Ascitic fluid is analyzed for:
Appearance
Albumin levels
Cell count
Total protein
Cell culture
The serum to ascites albumin gradient (SAAG) is used to determine if ascitic fluid is the result of portal hypertension. It is calculated by subtracting the ascitic fluid albumin level from the serum albumin level: SAAG = (serum albumin) - (ascitic albumin)
A serum to ascites albumin gradient greater than 1.1 g/dL indicates the ascites is due to portal hypertension. Possible causes of portal hypertension include:
Cirrhosis
Budd-Chiari syndrome
CHF
Constrictive pericarditis
Schistosomiasis
A serum to ascites albumin gradient less than 1.1 g/dL indicates an etiology other than portal hypertension such as nephrotic syndrome and cancer.
Compliations
Ascites is treated symptomatically through several strategies:
Sodium restriction
Diuretics such as furosemide (Lasix) or spironolactone (Aldactone)
Beta blockers to lower portal pressure in cirrhotic patients
Concerning complications of ascites include:
Spontaneous bacterial peritonitis
Hepatic hydrothorax
Gallbladder
Acute Cholecysitis
Acute cholecystitis is inflammation of the gallbladder caused by gallstone obstruction of the cystic duct.
Acute cholecystitis typically presents with:
Right upper quadrant or epigastric pain
May radiate to shoulder or back
Accompanied by abdominal guarding, nausea, vomiting, anorexia
Fever
Leukocytosis
Positive Murphy's sign
When the inflammation progresses to involve the parietal peritoneum, there is guarding and rebound tenderness.
Murphy’s sign is frequently associated with acute cholecystitis.
Murphy’s sign is inspiratory arrest with deep palpation of the right upper subcostal area. When inspiring, the diaphragm pushes the liver and gallbladder inferiorly, until it contacts the examiners palpations. The contact is painful, and the patient halts inspiration.
The pain in acute cholecystitis persists and worsens in contrast with chronic cholecystitis in which the pain is intermittent and only lasts a few hours.
Typical lab values include elevated:
WBC count
Liver enzymes
Bilirubin
The preferred method of diagnosing cholecystitis (acute, chronic and acalculous) is ultrasound.
Cholescintigraphy (HIDA scan) can help, and will show a lack of biliary filling due to the obstruction. Acute cholecystitis can be excluded if the HIDA scan is normal. Use HIDA if US is indeterminate.
The hepatobiliary iminodiacetic acid (HIDA) scan uses a nuclear tracer that is excreted in bile. Failure to visualize the tracer in the gallbladder suggests obstruction. HIDA can be used for evaluating cholecystitis in patients with indeterminate ultrasound findings.
The initial treatment for acute cholecystitis is IV fluids, antibiotics, and pain control.
Antibiotics used in the treatment of cholecystitis should cover the most common organisms (E. coli, Bacteroides fragilis, Klebsiella, Pseudomonas, Enterococcus), and include:
Ampicillin/sulbactam or pipercillin/tazobactam(non-life threatening cases)
Imipenem/cilastatin or meropenem (life threatening cases)
Alternatives include metronidazole + one of the following: 3rd generation cephalosporin OR ciprofloxacin OR aztreonam
Cholecystectomy is the definitive treatment, and should be performed within a few days of the illness if there are no other contraindications.
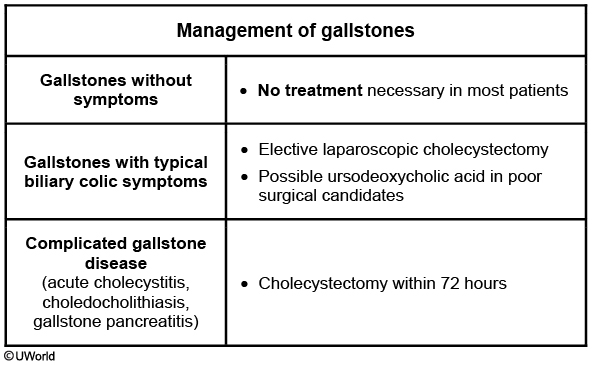
Symptoms often subside within a few days with volume resuscitation, antibiotics, and pain medications. However, early cholecystectomy (within 72 hours) reduces disease duration, duration of hospitalization, and mortality when compared to delayed cholecystectomy (>7 days after hospitalization)
Chronic cholecystitis
Chronic cholecystitis is defined as recurrent episodes of pain due to the cystic duct being obstructed by a gallstone.
The pain of chronic cholecystitis generally lasts a few hours.
Symptoms may include:
Right upper quadrant or epigastric pain, often radiating to the back and/or shoulder
Nausea
Vomiting
Lab tests (WBC count, liver function tests, bilirubin) are typically normal in chronic cholecystitis. The duct obstruction only lasts a few hours, until the stone passes and normal bile flow from the gallbladder is restored.
If the pain lasts 24 hours, acute cholecystitis and stones in the common bile duct should be considered.
The treatment for chronic cholecystitis is cholecystectomy. For patients who are poor surgical candidates or who desire a non-surgical option, treatment includes ursodeoxycholic acid and avoidance of fatty foods.
Acalculous Cholecystitis
Cholecystitis can occur without the presence of stones, and is called acalculous cholecystitis. Bile stasis and gallbladder ischemia are thought to be contributing factors to the development of acalculous cholecystitis.
This condition is most often seen in severely ill patients in the intensive care unit with multiorgan failure, severe trauma, surgery, burns, sepsis, or prolonged parenteral nutrition. Acalculous cholecystitis is likely due to cholestasis and gallbladder ischemia leading to secondary infection by enteric organisms and resultant edema and necrosis of the gallbladder. Most patients affected by this condition have no prior history of gallbladder disease.
The signs and symptoms of acalculous cholecystitis are similar to those of acute cholecystitis: right upper quadrant pain, leukocytosis, and fever. No jaundice as opposed to cholangitis.
Ultrasound is the preferred method of diagnosis, which often reveals a distended gallbladder, biliary sludge, and no stones.
Patients with acalculous cholecystitis require a percutaneous cholecystostomy (not cholecystectomy) since these patients are often not good surgical candidates.
Emphysema Cholecystitis

This patient with fever, right upper quadrant (RUQ) pain, and gas in the gallbladder wall is presenting with clinical manifestations of acute emphysematous cholecystitis, a life-threatening form of acute cholecystitis due to infection with gas-forming bacteria (eg, Clostridium, some Escherichia coli strains). Predisposing factors include vascular compromise (obstruction or stenosis of the cystic artery), immunosuppression (eg, diabetes mellitus), and gallstones. Crepitus in the abdominal wall adjacent to the gallbladder is occasionally detectable. Complications include gangrene and perforation, the latter of which may transiently relieve pain but subsequently result in peritoneal signs.
Diagnosis is confirmed with imaging demonstrating air-fluid levels in the gallbladder (as gas leaks into it), gas in the gallbladder wall, and occasionally pneumobilia (air within the hepatobiliary system). Laboratory findings include mild to moderate unconjugated hyperbilirubinemia (eg, from Clostridium-induced hemolysis) and a small elevation in aminotransferases. Treatment requires emergent cholecystectomy and broad-spectrum parenteral antibiotic therapy (eg, ampicillin-sulbactam).

Fitz-Hugh Cutis
Fitz-Hugh Curtis syndrome (perihepatitis in the setting of pelvic inflammatory disease) is associated with RUQ pain that worsens with inspiration. Imaging would show inflammation of the hepatic capsule but no air in the gallbladder wall.
Sphincter of Oddi Dysfunction
This patient likely has postcholecystectomy pain due to sphincter of Oddi dysfunction (SOD). The sphincter of Oddi is a muscular valve controlling the flow of bile and pancreatic juice into the duodenum.
SOD, which can develop following any inflammatory process (eg, surgery, pancreatitis), encompasses 2 separate physiologic entities: dyskinesia and stenosis of the sphincter of Oddi. Obstruction of flow through the sphincter may result in retention of bile, causing a functional biliary disorder that mimics a structural lesion. Recurrent, episodic pain in the right upper quadrant or epigastric region, with corresponding aminotransferase and alkaline phosphatase elevations, is common; visualization of a dilated common bile duct in the absence of stones increases the likelihood of SOD. Opioid analgesics (eg, morphine) may cause sphincter contraction and precipitate symptoms of SOD, as seen in this patient (worse pain with morphine).
Sphincter of Oddi manometry is the gold standard for the diagnosis of SOD; sphincterotomy is the treatment of choice in most cases.
Choledocholithiasis
Choledocholithiasis is a specific subset of cholelithiasis in which gallstones are found in the common bile duct.
Choledocholithiasis often results in biliary colic (post-prandial right upper quadrant pain, may be waxing and waning in nature), jaundice, light-colored stools, and dark urine.
Jaundice results because the bile drainage is blocked.
Stools are light because bilirubin metabolism in the gut is what results in its dark color. Without bile excretion into the gut, bilirubin does not contribute to the stool color.
The urine is dark/tea-colored because the excessive bilirubin in the body is absorbed into the blood, carried to the kidneys, and excreted in the urine.
For choledocholithiasis, the initial diagnostic method is transabdominal ultrasound, used to risk stratify patients for likelihood of choledocholithiasis. The following stratification is according to the American Society of Gastrointestinal Endoscopy guidelines:
Low-risk patients with symptomatic cholelithiasis should undergo cholecystectomy
Intermediate-risk patients undergo preoperative ultrasound or MRCP to triage the need for ductal stone clearance
High-risk patients require further evaluation of the common bile duct, which may include ERCP or operative cholangiography
Choledocholithiasis can lead to cholangitis and gallstone pancreatitis.
Once a diagnosis of choledocholithiasis is made, the stones are removed via an endoscopic retrograde cholangiopancreatography (ERCP), followed by an elective cholecystectomy.
Acute cholangitis
Acute cholangitis is an ascending bacterial infection of the common bile duct. It is a complication of choledocholithiasis.
The most common contributing organisms are:
E. coli (25 to 50%)
Klebsiella pneumonia (15 to 20%)
Enterobacter species (5 to 10%)
Enterococcus species (10 to 20%)
Bacteroides and Clostridia (anaerobes, usually part of a mixed infection)
Acute cholangitis is associated with Charcot’s triad: fever, right upper-quadrant pain, and jaundice. Jaundice more common than in cholecystitis.
Reynolds' pentad indicates suppurative cholangitis, a complication of acute cholangitis. It consists of the same three criteria of Charcot’s triad, plus the addition of altered mental status and hypotension due to septic shock.
In addition to clinical findings, acute cholangitis often results in elevated levels of:
WBCs
Bilirubin
Alkaline phosphatase
Transaminases (AST, ALT)
Imaging would show bild duct dilation without gas in biliary tree as opposed to emphysematous cholecystitis.
The initial treatment of acute cholangitis is IV fluids and antibiotics.
If the patient is unable to be managed via IV fluids and antibiotics, the following procedures may be considered:
Decompression and drainage of biliary system
Endoscopic drainage
Percutaneous transhepatic biliary drainage (PTBD)
Surgical decompression if endoscopic procedure is unsuccessful
Cholelithiasis
Cholelithiasis is the presence of gallstones in the gallbladder.
Most (75%) of gallstones are cholesterol stones, with pigment stones making up the rest (25%).
When bile is oversaturated with cholesterol, the cholesterol precipitates and forms solid crystals.
Black stones are a sign of hemolysis. Brown stones are a sign of infection of the biliary tract.
Risk factors for cholelithiasis include:
Obesity
Female gender
Increased age
Increased estrogen exposure
Same risk factors for the development of biliary sludge(total parenteral nutrition, rapid weight loss, and starvation)
Remember the 4 F’s: Fat, forty, female, fertile.
Cholelithiasis is asymptomatic in as many as 80% of patients, and is found incidentally.
When symptomatic, cholelithiasis often presents with:
Biliary colic in the right upper quadrant, often following a meal (due to gallbladder contraction)
Pain radiating to the back and/or shoulder
Nausea and vomiting
Restlessness due to pain (in contrast to cholecystitis, where patients lie still because movement exacerbates the pain)
Aside from a thorough physical and history, the best diagnostic test for cholelithiasis is ultrasound. Ultrasound identifies over 95% of gallstones.
Complications of cholelithiasis include:
Recurrent gallstones
Acute cholecystitis (See topic here: Gallbladder: Cholecystitis)
Pancreatitis (See topic here: Pancreatitis: Acute)
Gallstone ileus
Gallstone Ileus
In gallstone ileus, stones that erode through the gallbladder into the small bowel can cause obstruction, especially in the ileum.

Cholecystitis, which predisposes to biliary-enteric adhesions, is the most important risk factor, and patients are more commonly elderly women, which reflects their higher prevalence of gallstone disease.
Symptoms of gallstone ileus include:
Abdominal pain
Abdominal distension
Nausea
Vomiting
Hypovolemia
Jaundice
Weight loss/anorexia
Imaging findings of gallstone ileus include:
Air in the hepatic bile ducts
Small bowel obstruction with an air-fluid level
Gallstones in the ileocecal valve
CT scan is the preferred test for gallstone ileus. It can show all of the features of gallstone ileus, including the fistula between the gallbladder and intestine. Abdominal X-ray may reveal air in the biliary tract and an air-fluid level in the ileus.
Treatment of gallstone ileus is with enterotomy to remove the stone. In addition, the bowel proximal to the stone should be examined for stones that might result in symptoms later.
Once the patient has recovered from enterotomy, a cholecystectomy should be scheduled if there are still symptoms of gallstone disease.
Cholestasis
This patient’s diagnosis is cholestasis of pregnancy. The most common symptom is intense pruritus, which may be generalized but often is worse at night and on the palms and soles, elevated serum bile acids, and elevated liver enzymes. Serum total bile acids may be the only laboratory abnormality noted, although liver aminotransferases can be significantly elevated. Patients will also have elevated serum bile acids of over 10 micromol/L. Ursodeoxycholic acid, which reduces bile production, has shown to be effective in treating the condition. Although the maternal prognosis for cholestasis of pregnancy is good, with resolution after delivery, cholestasis of pregnancy carries significant risk for the fetus. Risks include intrauterine fetal demise, neonatal respiratory distress syndrome, and meconium stained amniotic fluid. Antenatal testing has not been definitively proven to decrease the risk of stillbirth, but many obstetricians will perform at least weekly fetal antenatal testing and laboratory evaluation. Once the diagnosis of cholestasis is confirmed, the recommendation is for delivery at 36 weeks.
Hiatal Hernia
A hiatal hernia occurs when the upper portion of the stomach protrudes through the esophageal hiatus of the diaphragm.
In general, there are 2 types of hiatal hernias, sliding (most common) and paraesophageal.
A type I (sliding hernia) occurs when both the gastroesophageal junction and proximal stomach herniate upwards.
A type 2 (paraesophageal) hernia occurs when only the proximal stomach herniates, while the gastroesophageal junction remains in place.

Incidence increases with age (50% over age 50).
The majority of patients with hiatal hernias are asymptomatic. When symptoms are present, the most common symptoms include:
Acid reflux
Chest pain
Nausea
Early satiety
Upper endoscopy is the best tool for diagnosis of a hiatal hernia. It will reveal retrograde movement of the stomach.
Some hiatal hernias are discovered incidentally on chest x-ray, but a barium study of the esophagus is much more accurate.
Hiatal hernias may result in the following complications:
Acid reflux
Esophagitis
Esophageal strictures
Perforation
Volvulus
Strangulated hernia pouch
Paraesophageal hernias have a much higher risk of strangulation and volvulus formation.
When patients are symptomatic, treatment primarily consists of proton pump inhibitors (PPI) and dietary modifications such as eating smaller portions and increasing fiber intake.
Patients who are refractory to medical therapy or present with a volvulus should undergo a Nissen fundoplication. This procedure involves a 360-degree wrap of the fundus around the gastroesophageal junction to help prevent herniation.
Acute Pancreatitis
Causes
Acute pancreatitis is an inflammatory condition, which results in severe abdominal pain along with elevated pancreatic enzymes. Though the exact pathogenesis is not well known, there are several etiologies that have been linked to acute attacks.
Biliary tract disease (i.e. gallstones) and chronic alcohol use are the two most common causes of acute pancreatitis.
The various causes of acute pancreatitis can be remembered with the mnemonic GET SMASHED:
Gallstones: Obstruction of duct → back-up into pancreas. Some studies have shown that ALT >150 U/L has a 95% positive predictive value for diagnosing gallstone pancreatitis.
Ethanol: Damage to acinar cells, thickening of ductal secretions → obstruction
Trauma: Blunt abdominal trauma can cause mechanical damage to pancreas
Steroids
Mumps
Autoimmune
Scorpion sting
Hypercalcemia/Hypertriglyceridemia: Metabolic activation of enzymes (Ca) or chemical injury (TGs); Hereditary
ERCP (endoscopic retrograde cholangiopancreatography procedure)
Drugs (esp. sulfa): Chemical injury to acinar cells
Diuretics (furosemide, thiazides)
Drugs for inflammatory bowel disease (sulfasalazine, 5-ASA)
Immunosuppressive agents (azathioprine)
HIV-related medications (didanosine, pentamidine)
Antibiotics (metronidazole, tetracycline)
Symptoms
Patients with acute pancreatitis will typically present with severe epigastric abdominal pain that radiates straight to their back, nausea, vomiting, and inability to get comfortable on the examination table.
Depending on the etiology, patients will usually present to the hospital within 24-48 hours of the event.
On physical exam patients will often have tachycardia, fever, vomiting, severe tenderness in the epigastric area, and pale mucosa.
Other signs to watch for include: dyspnea, hypotension, and bruising around the umbilicus (Cullen sign) and flanks (Grey-Turner sign), which is seen in hemorrhagic pancreatitis.
Diagnosis
When acute pancreatitis is suspected, the best initial step is to obtain amylase and lipase levels. These levels are typically 3-4 times the upper limit, with lipase being more sensitive and specific for acute pancreatitis.
Other laboratory tests, which may be elevated, include:
ALT, AST
Bilirubin
Alkaline phosphatase
Calcium (unlike the other lab values here, will be decreased)
BUN/Cr
Triglycerides
WBC count
In patients with a clinical diagnosis of pancreatitis, imaging is not typically required for confirmation. Imaging should be performed in patients where:
The pancreatitis is severe
Tumor is suspected
There is suspicion for duct obstruction
Contrast-enhanced computed tomography (CECT) is the standard imaging modality for the evaluation of acute pancreatitis and its complications, including necrosis and vascular complications. Non-contrast CT scan can be used, however, it cannot assess for necrosis or vascular complications.
Ultrasound is used to look for gallstones, biliary sludge or a blockage.
Once stones and a dilated common bile duct are detected on ultrasound, endoscopic retrograde cholangiopancreatography (ERCP) may be used to confirm a diagnosis of choledocholithiasis and also for therapeutic purposes by removing the stone(s) responsible for symptoms.
Ranson's criteria are commonly used to estimate the mortality of patients with pancreatitis based on initial and 48 hour lab values. They include:
On Admission:
Leukocytosis (WBC count of >16,000)
Enzyme AST >250
Glucose >200 mg/dL
Age >55
LDH >350
48 hours after admission:
Ca < 8 mg/dL
Hct drop >10%
Oxygen needs (Arterial pO2 < 60 mmHg)
BUN increase > 5 mg/dL
Base deficit > 4 mg/dL
Sequestration of fluid (defined by fluid needs > 6 L)
Each additional criterion a patient has coincides with an increased risk of mortality.
A mnemonic for remembering the admission values for Ranson's criteria: LEGAL (Leukocytosis, Enzyme AST, Glucose, Age, LDH)
To remember the 48 h values, think about Calvin and Hobbes (C & HOBBS): Calcium, Hct drop, Oxygen, BUN, Base deficit, Sequestration of fluid
Treatment
Treatment of acute pancreatitis consists of:
IV fluid resuscitation
NPO (nil per os, nothing by mouth)
Analgesics (e.g. fentanyl)
Pharmacologic pain control consists of IV hydromorphone, fentanyl, or meperidine. Empiric antibiotics are not used, unless pancreatic necrosis is suspected.
Surgical intervention is typically not required in the treatment of acute pancreatitis, but drainage may be performed if an abscess or pseudocyst is present.
ERCP is incorporated when gallstone pancreatitis is suspected. This procedure uses an endoscope to dilate and remove stones within the pancreatic duct.
Complications
The most high-yield complications of acute pancreatitis are:
Necrotizing pancreatitis
Abscess
Pseudocyst
Early acute pancreatitis complications: GRASS
Glucose elevated
Renal failure
ARDS
Sepsis
Shock
Late acute pancreatitis complications: NAP
Necrosis
Abscess
Pseudocyst
Pancreatic Carcinoma

This patient has epigastric pain/tenderness and weight loss in the setting of nonspecific systemic symptoms and a significant smoking history. This combination strongly suggests a malignancy affecting the upper gastrointestinal tract or associated solid organs, such as the liver, gallbladder, or pancreas. Among the choices listed, pancreatic adenocarcinoma is the most likely diagnosis.
Adenocarcinoma of the pancreas is the fourth leading cause of cancer-related death in the United States and has a mean age of 55 at diagnosis. Smoking is an important risk factor. Pancreatic cancer has a very high mortality rate as it is frequently diagnosed at relatively late stages (early-stage disease usually causes only mild, nonspecific symptoms).
Symptoms of pancreatic cancer vary and depend primarily on the location of the tumor. Most (60%-70%) pancreatic cancers originate in the head of the pancreas. These cancers are more likely to present with jaundice (common bile duct obstruction) and steatorrhea (inability to secrete fat-digesting enzymes or blockage in the main pancreatic duct). However, jaundice may appear at a later stage if the tumor arises from the tail or body of the pancreas. Most patients describe epigastric abdominal pain that is usually insidious, gnawing, and worse at night. However, some patients may not have pain and simply present with painless jaundice. Systemic symptoms such as weight loss and fatigue are common. Another classic association is migratory thrombophlebitis (Trousseau sign).
Diagnosis is established with either ultrasound (less expensive) in patients with jaundice (head tumors) or CT scan (highly sensitive) in patients without jaundice (body and tail tumors).
Subphrenic Abscess
A subphrenic abscess is a collection of infected fluid located inferior to the diaphragm, between the liver and the spleen. It can develop post-operatively after abdominal procedures.
Subphrenic abscesses form slowly and manifest approximately 1 month after an abdominal surgery.
Patients may present with:
Fever
Abdominal pain
Weight loss
Nausea/vomiting
Shoulder pain (secondary to diaphragmatic irritation)
Subphrenic abscesses can be detected by imaging (ultrasound, plain film, or CT). Chest X-ray may show a pleural effusion.


CBC may show a leukocytosis.
The treatment of these abscesses depends on careful drainage and broad-spectrum IV antibiotics.
Caution must be used since perforation or leakage during aspiration may cause seeding or subsequent peritonitis.
Volvulus
A volvulus involves twisting of the intestine around its mesenteric root causing obstruction to the bowel lumen as well as to the lymphatic and venous drainage. Eventually, the arterial supply is compromised resulting in gut ischemia and necrosis. It is a surgical emergency.
Because congenital malrotation predisposes to midgut volvulus, when diagnosed, it is usually repaired surgically to avoid the potentially life-threatening development of a volvulus.
In infancy, the most common presentation of volvulus is abdominal distention with acute onset of bilious vomiting. Other signs and symptoms include:
Abdominal pain
Constipation
Hematochezia (10-15% of patients, suggests the presence of bowel ischemia)
On KUB (kidney, ureter, bladder) radiographs, a classic "coffee bean sign" may be seen, indicating a sigmoid volvulus.
In the presence of a volvulus, the distal duodenum and proximal jejunum course downward in the mid abdomen. On contrast radiography, this may be seen as a “corkscrew” appearance. The lumen also becomes narrowed and may have a “beaked” appearance at the level of obstruction.
sigmoid colon:

If volvulus is suspected in a pediatric patient, the patient should be managed by:
Making them NPO
Placing a nasogastric tube
Giving appropriate resuscitation
Immediate surgical intervention is then indicated.
Complications of volvulus include:
Obstruction
Necrosis
Perforation
Recurrent volvulus
Zenker Diverticulum
Zenker diverticulum is an outpouching of the upper, posterior esophagus in a natural area of weakness known as Killian's triangle that is caused by increased intrabolus pressures during swallowing.
The classic presentation of Zenker diverticulum is the regurgitation of food that was eaten days before. Why? The food gets caught in the diverticulum itself, and remains there until regurgitated.
Other symptoms of Zenker diverticulum include:
Bad breath
Problems initiating swallowing
Neck mass that increases with size during eating and drinking (from contents filling the diverticulum)
Feelings of aspiration
Dysphagia
Zenker diverticulum is diagnosed with a barium swallow, which will show the outpouching of the esophagus.
These patients are more prone to an esophageal rupture during EGD because the esophageal wall is weaker.
Zenker diverticulum is typically treated surgically with a crycopharyngeal myotomy or a diverticulectomy.
Complications of surgical treatment include vocal cord paralysis and mediastinitis.
Pilonidal Cyst

PD most frequently affects individuals age 15-30, particularly young males, obese individuals, those with sedentary lifestyles or occupations, and those with deep gluteal clefts. The most common presenting manifestations include a painful, fluctuant mass 4-5 cm cephalad to the anus in the intergluteal region with associated mucoid, purulent, or bloody drainage. Pain is frequently worsened by activities that stretch the overlying skin (eg, bending down).
PD develops when an edematous, infected hair follicle in the intergluteal region becomes occluded. The infection spreads subcutaneously and forms an abscess, which can rupture and create a pilonidal sinus tract. As the patient sits or stands, hair and debris are forced into the sinus tract, resulting in recurrent infections and foreign-body reactions. Treatment is drainage of abscesses and collected debris followed by excision of sinus tracts. Despite longer healing times, open closure is preferred due to decreased recurrence rates.
perianal abscess has fever. Bulge is at anal verge
pilonidal cyst: no fever, bulge superior to anus
Acute Abd Pain in Women

This patient presents with hemoperitoneum due to a ruptured ovarian cyst. Corpus luteum cysts form in the second half of the menstrual cycle, after ovulation has occurred. Although hemoperitoneum does not typically occur with ovarian cyst rupture, patients who are on anticoagulation can bleed intra-abdominally and become hemodynamically unstable. Typical presentation of a ruptured cyst is sudden onset of unilateral lower abdominal pain. Symptoms of hemoperitoneum include diffuse severe abdominal pain, pleuritic chest pain, and shoulder pain (due to phrenic nerve irritation). Physical examination with a ruptured ovarian cyst shows unilateral lower quadrant tenderness; with hemoperitoneum, diffuse abdominal rigidity with rebound and guarding is present. Laboratory testing shows a decreased hematocrit due to intra-abdominal blood loss. Pelvic ultrasound confirms intra-abdominal and pelvic free fluid and a possible adnexal mass (if the cyst is incompletely drained). Treatment of a hemodynamically unstable patient with hemoperitoneum due to a ruptured ovarian cyst is surgery to stop the bleeding.
Ovarian torsion may present with sudden lower abdominal pain; it does not typically result in an acute abdomen on physical examination and does not cause a drop in hematocrit.
Viscus Perforation
This patient with acute-onset severe abdominal pain, fever, tachycardia, and signs of peritonitis (eg, guarding, rigidity, reduced bowel sounds, rebound tenderness) likely has a perforated viscus. Her preceding episodic epigastric pain, nausea, history of NSAID and alcohol use, and positive stool guaiac test raise suspicion for peptic ulcer disease as the cause of perforation. The diagnosis of gastrointestinal perforation is confirmed with upright x-ray of the chest and abdomen, which typically shows free intraperitoneal air under the diaphragm (pneumoperitoneum).

Dumping Syndrome

This patient has clinical features typical of early dumping syndrome (DS), including gastrointestinal (eg, nausea, diarrhea, abdominal cramps) and vasomotor (eg, palpitations, diaphoresis) symptoms. DS is a common postgastrectomy complication occurring in up to 50% of patients. It is caused by loss of the normal action of the pyloric sphincter due to injury or surgical bypass and leads to rapid emptying of hypertonic gastric contents into the duodenum and small intestine. This causes fluid shifts from the intravascular space to the small intestine, leading to hypotension, stimulation of autonomic reflexes, and release of intestinal vasoactive polypeptides.
The diagnosis of DS is primarily based on clinical features, although an upper gastrointestinal x-ray series or gastric emptying studies may be helpful if the diagnosis is unclear. Most patients can be managed with dietary modification:
Consume frequent, small meals and eat slowly
Avoid simple sugars
Increase fiber and protein
Drink fluids between rather than during meals
Symptoms of DS usually diminish over time. A minority of patients with refractory symptoms may benefit from a trial of octreotide or require reconstructive surgery, but this is not usually needed.
Gastric Outlet
This patient presents with signs/symptoms consistent with gastric outlet obstruction caused by mechanical obstruction, leading to postprandial pain and vomiting with early satiety. Common causes of gastric outlet obstruction include gastric malignancy, peptic ulcer disease, Crohn disease, strictures (with pyloric stenosis) secondary to ingestion of caustic agents, and gastric bezoars.
Physical examination can show an abdominal succussion splash, which is elicited by placing the stethoscope over the upper abdomen and rocking the patient back and forth at the hips. Retained gastric material >3 hours after a meal will generate a splash sound and indicates the presence of a hollow viscus filled with both fluid and gas.
This patient ingested acid 3 months ago, which is a risk factor for the development of a pyloric stricture. Acid ingestion causes fibrosis 6-12 weeks after the resolution of the acute injury. Upper endoscopy is usually required to confirm the diagnosis, and treatment is primarily surgical.
Sliding Hernia
Sliding inguinal hernias have a much higher risk of colonic injury during repair than other hernias. This is because the posterior wall of the hernia sac is formed by a retroperitoneal organ (colon or bladder). A clue to the presence of a sliding hernia is the finding of a thickened posterior wall of the hernia sac at surgery, in association with a large indirect hernia (D) that has descended into the scrotum (direct hernias rarely descend into the scrotum). Attempting to completely excise the hernia sac (A) (which is otherwise normally done), or to divide the sac completely at the internal ring (E) (which is again normally recommended), would result in dividing the bowel or bladder. Sliding hernias are more common on the left side (C) (the sigmoid colon is less fixed and more likely to slide down than the right colon). A sliding hernia is an indirect inguinal hernia (D).
Femoral Hernia
Multigravida causes stretching of the abdominal musculature and increases the risk of femoral hernia. Femoral hernias occur in the femoral canal, inferior to the inguinal ligament traversing the empty space medial (C) to the femoral vein (recall the mnemonic “NAVEL” {from lateral to medial: femoral nerve, artery, vein, empty space, lymphatic}). The most common type of hernia in women, and in men, is an indirect inguinal hernia (A). Although femoral hernias appear infrequently (10 % of all hernias), they occur more commonly in females and have the highest risk of strangulation (B). Because of the high risk of strangulation, surgical repair of a femoral hernia is indicated (D) once diagnosed, regardless of whether the patient is having symptoms.
Richter's Hernia
With a Richter’s hernia, only one wall of the bowel protrudes into the hernia sac (A). That segment of bowel is prone to incarceration and strangulation but does so without associated symptoms, signs, or radiologic evidence of SBO (C). Therefore, it may easily mislead clinicians into thinking that the hernia is not incarcerated (B). Manual reduction of hernias (including Richter’s) should not be attempted if strangulation is suspected as dead bowel will be reduced into the peritoneum. Strangulation should be suspected in the presence of fever, leukocytosis, acidosis, severe pain, or marked erythema overlying the skin of the hernia. It is often difficult to palpate a Richter’s hernia, and it should be reduced in the operating room (E).

Umbilical Hernia
It is recommended that repair be delayed (A) until after the child is 4 years old, unless the defect is larger than 2 cm, the defect is growing, or there is evidence of strangulation.
Last updated