03 Hemoglobin
_..
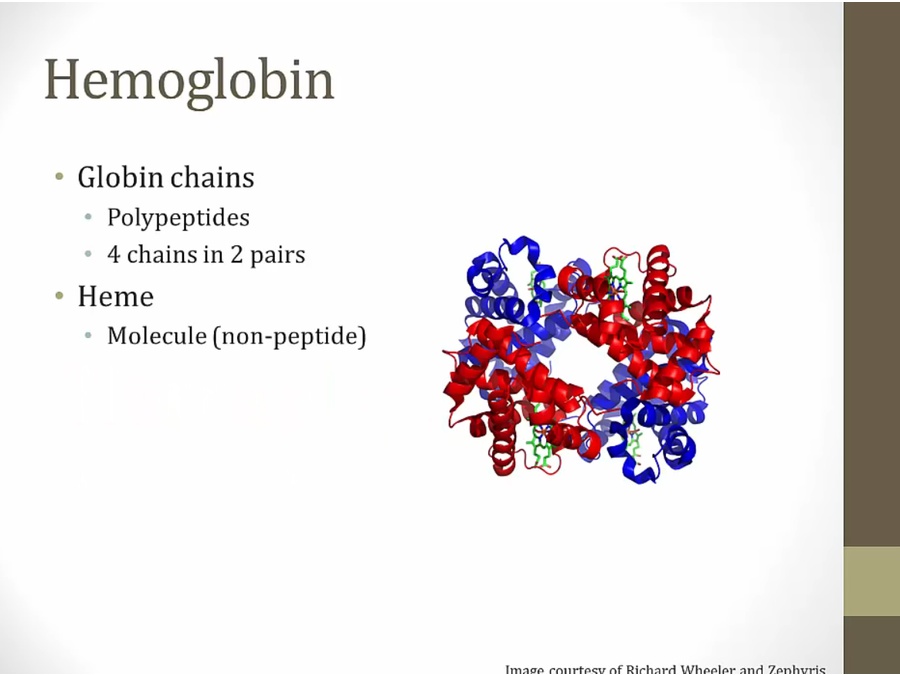
component of RBC, carries O2
blue and red: globin chains
green: heme, 4 in a hemoglobin
Globin
_..




sickle cell: abnormal hemoglobin

Heme
_..

porphyria: defects in enzymes making the porphyrin

_..


RBC with BPG Mutase makes 2, 3 BPG
bad for red cells: sacrifices ATP (pyruvate normally used to make ATP)
Hemoglobin Binding
_..

favored in tissues to release O2. T for tissues
relaxed form favored in lungs to pick up more O2

_..

myoglobin: bind each O2 molecule equally, linear increase until plateau
hemoglobin: S shaped curve. Hard to bind first one and then speeds up

example of allosteric effect
Allosteric Effects
_..





CO
_..


loses cooperativity
_..


MetHB
_..

rest of Fe2 binds O2 with higher affinity, left shift curve
_..

methylene blue reduces Fe3 to Fe2
vitamin C reduces Fe3 to 2

SOB from methemoblobinemia
interferes with pulse ox
normal amount of O2 in blood
CN
_..

functional hypoxia: enough O2 around but can't use them
inhibits cytochrome oxidase a3

Last updated