08 Acid Base Principles
Overview
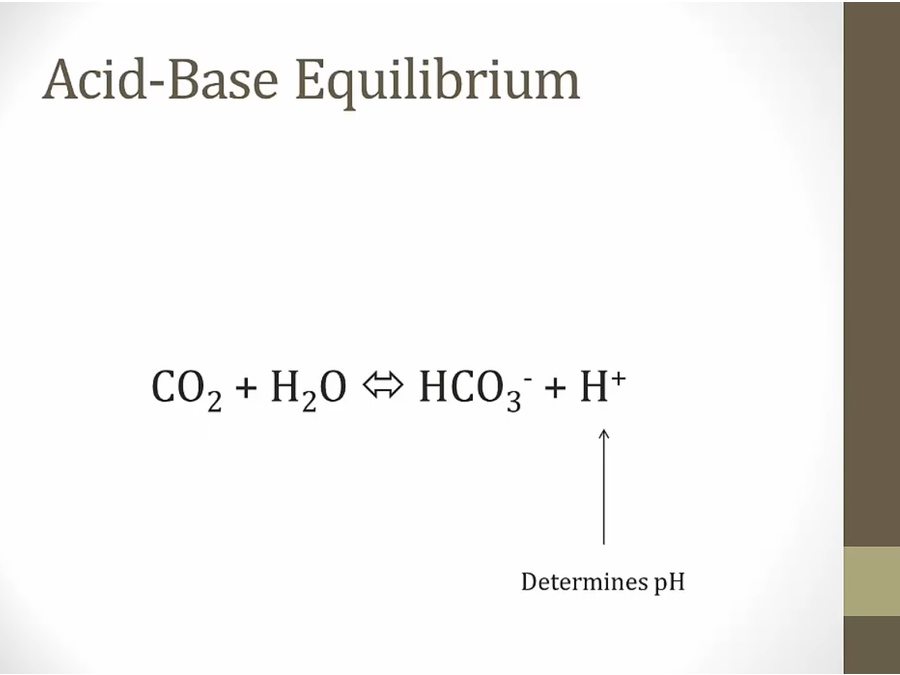
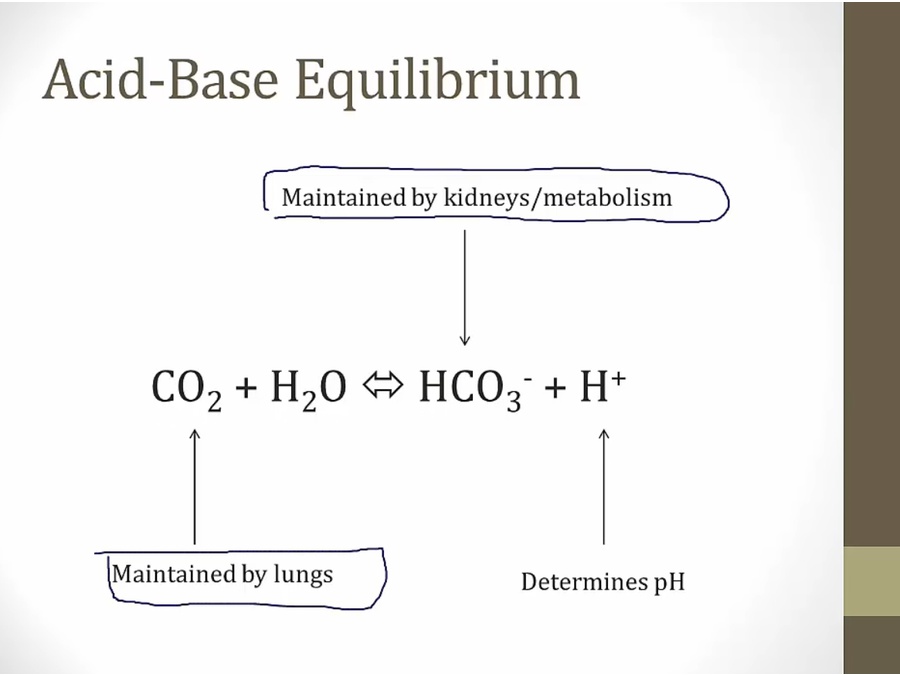
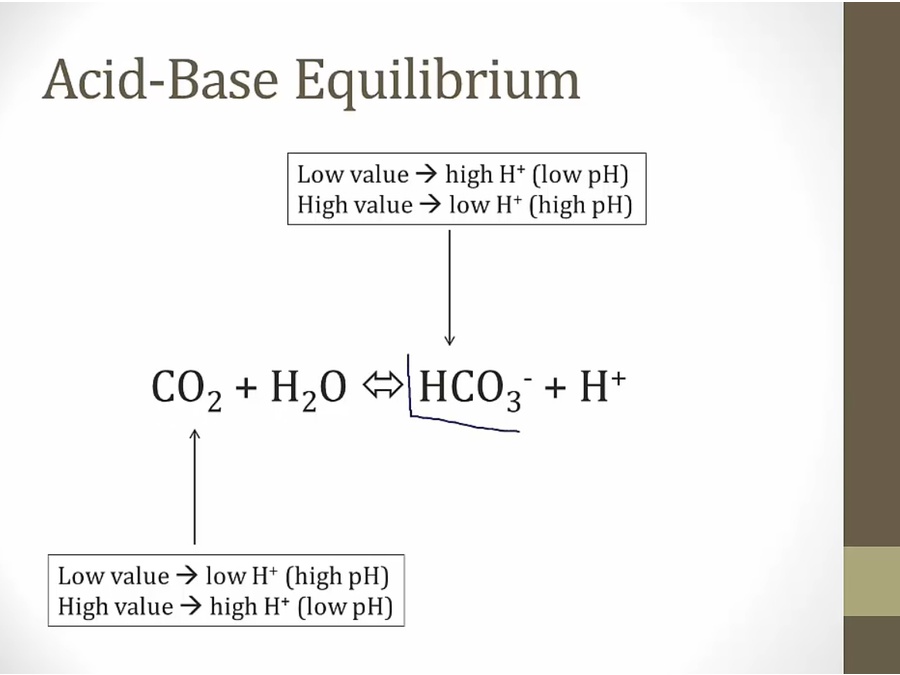
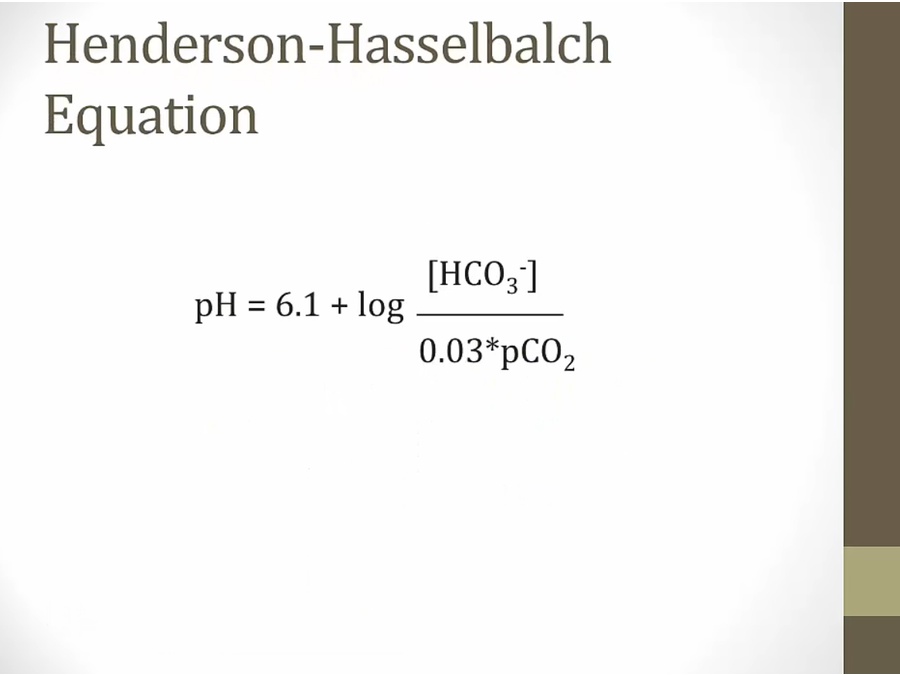
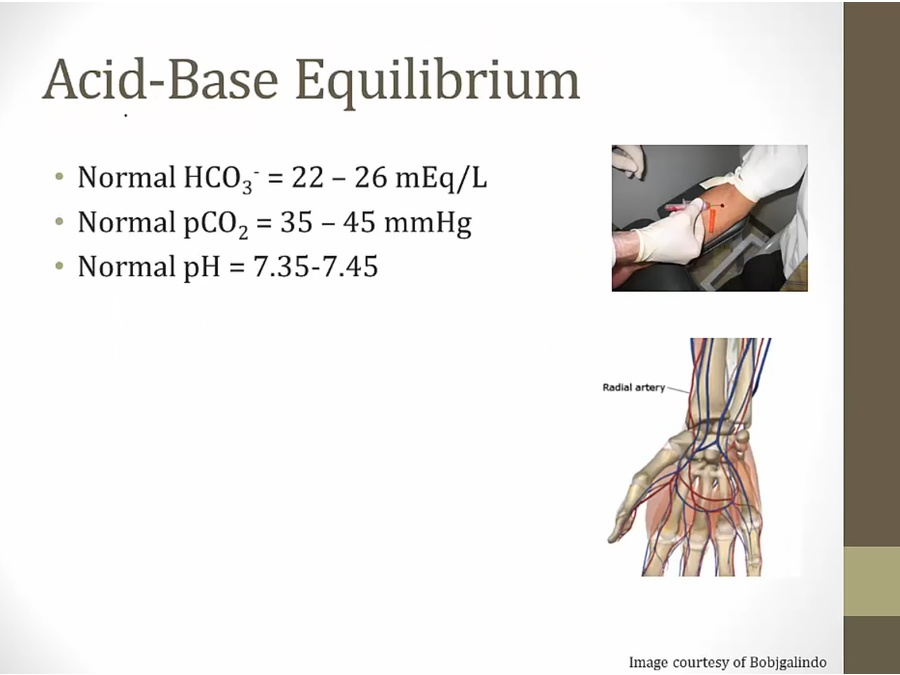

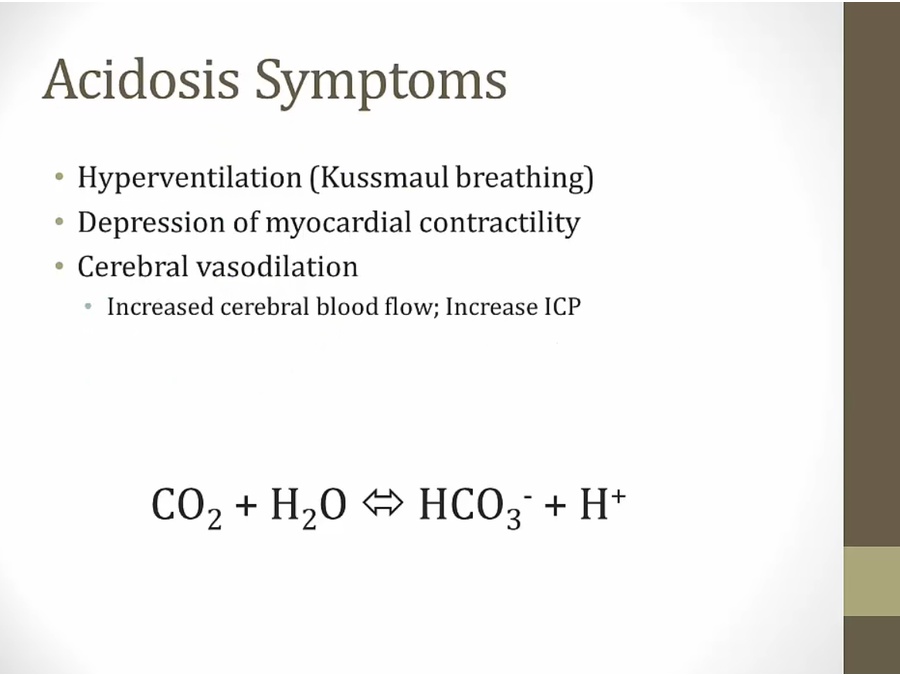
Acidosis

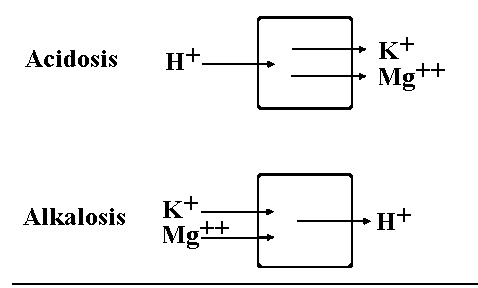
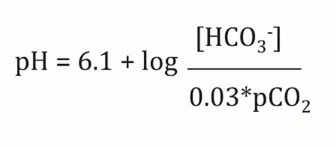
Alkalosis

Acid Base Problems
Examples
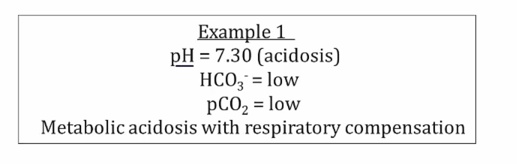
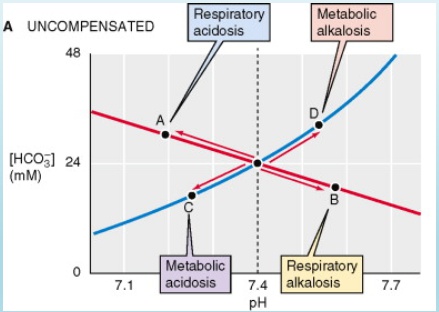
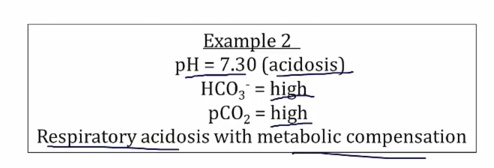

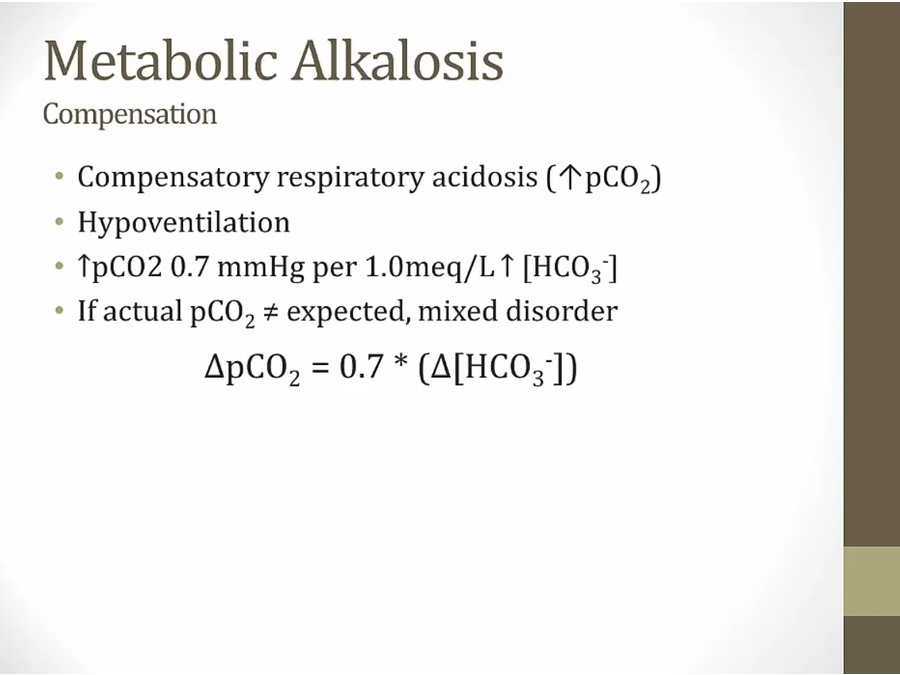
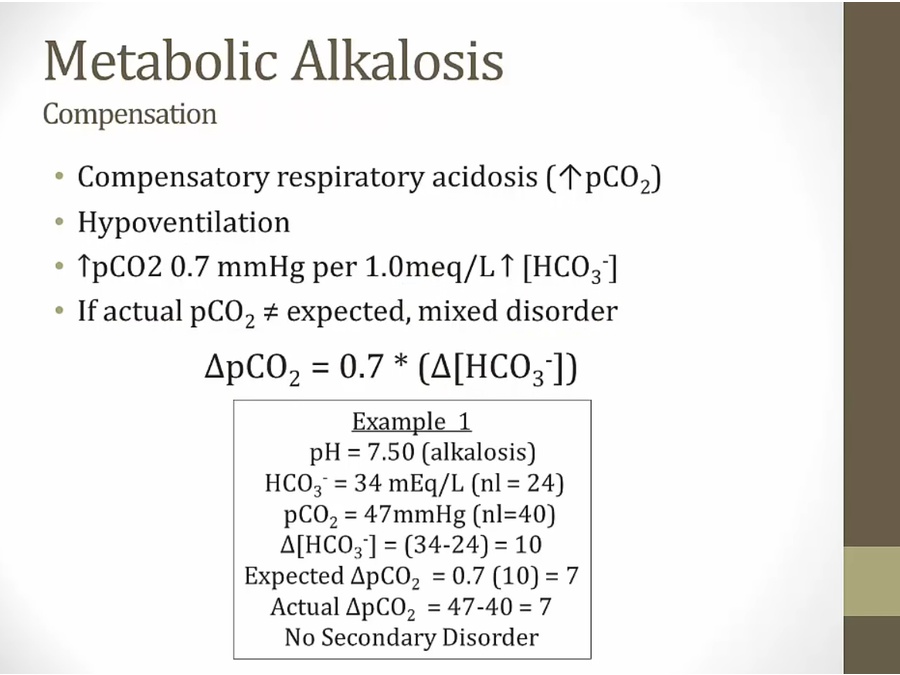
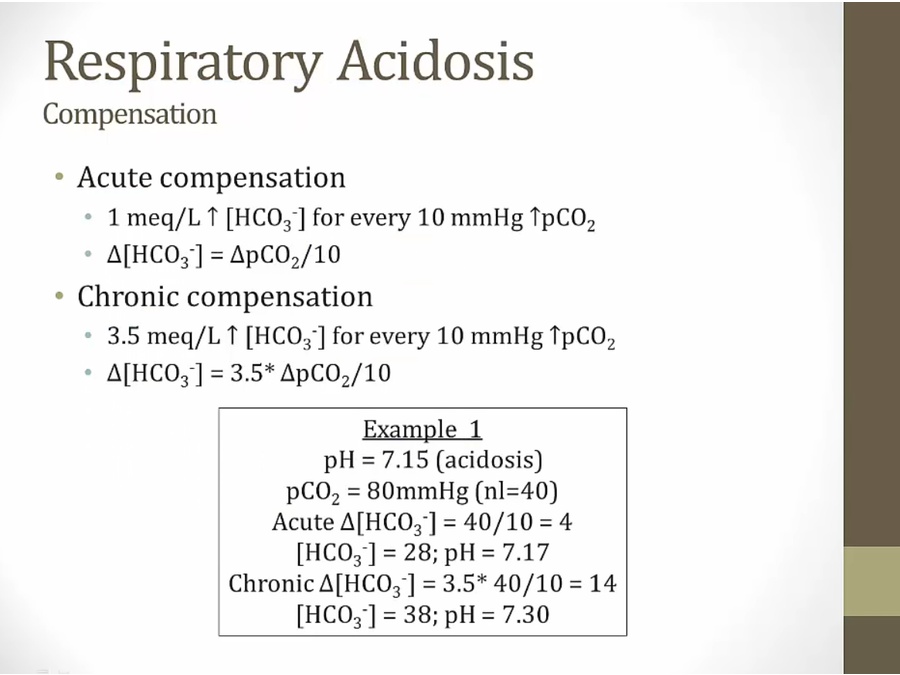
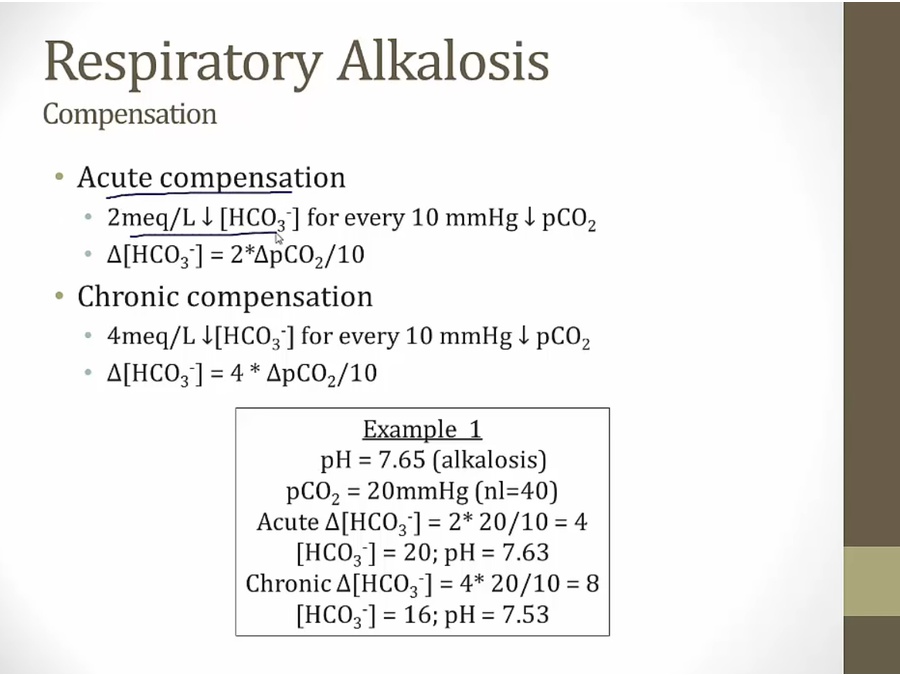
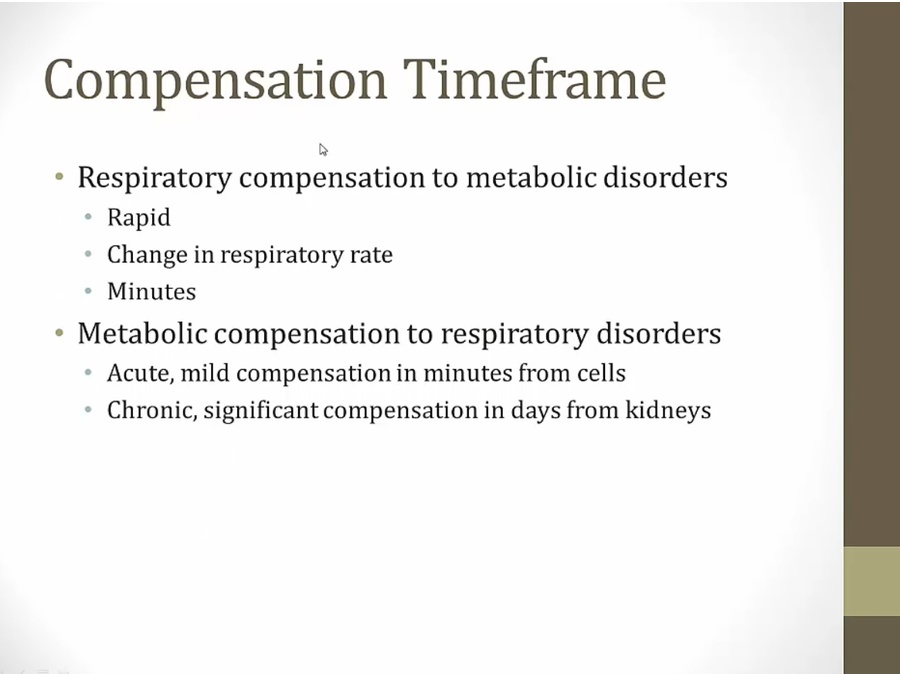
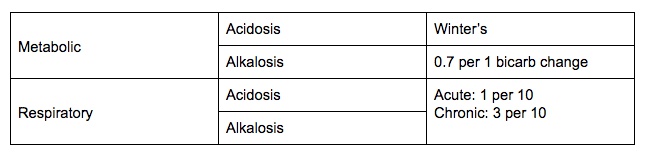
Compensation

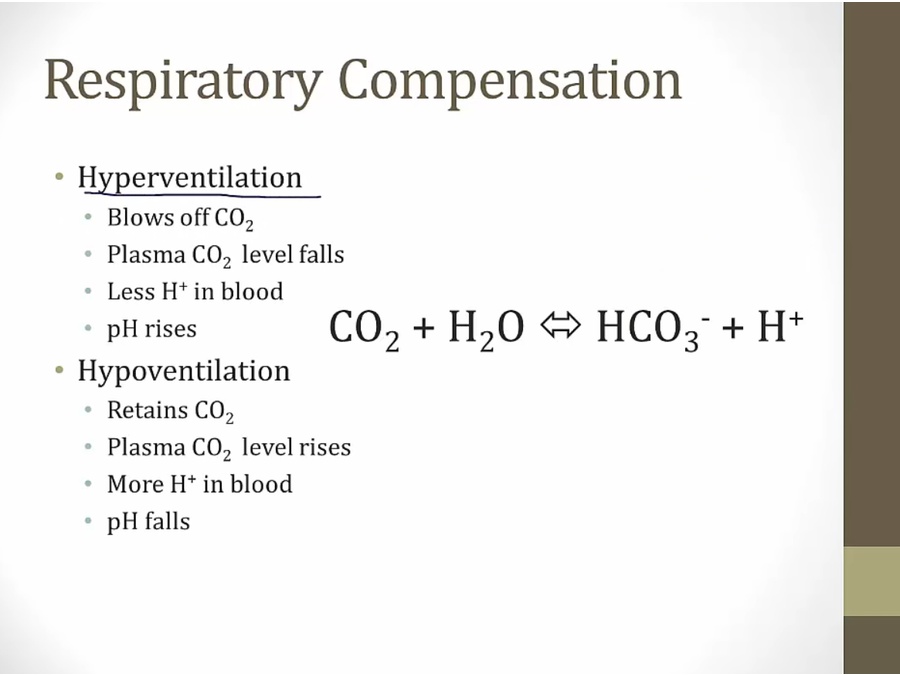


Mixed Disorders

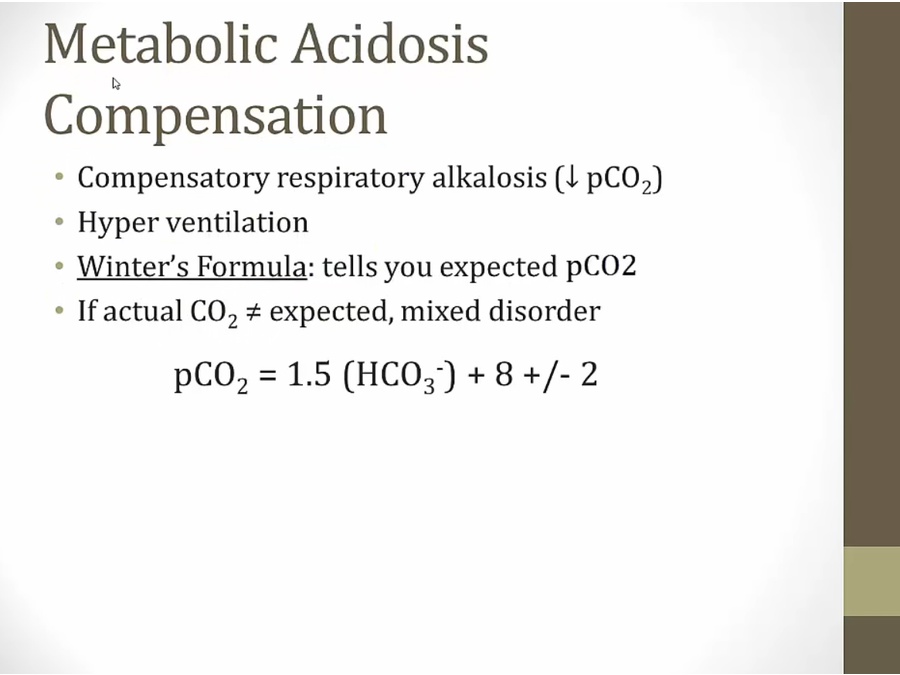
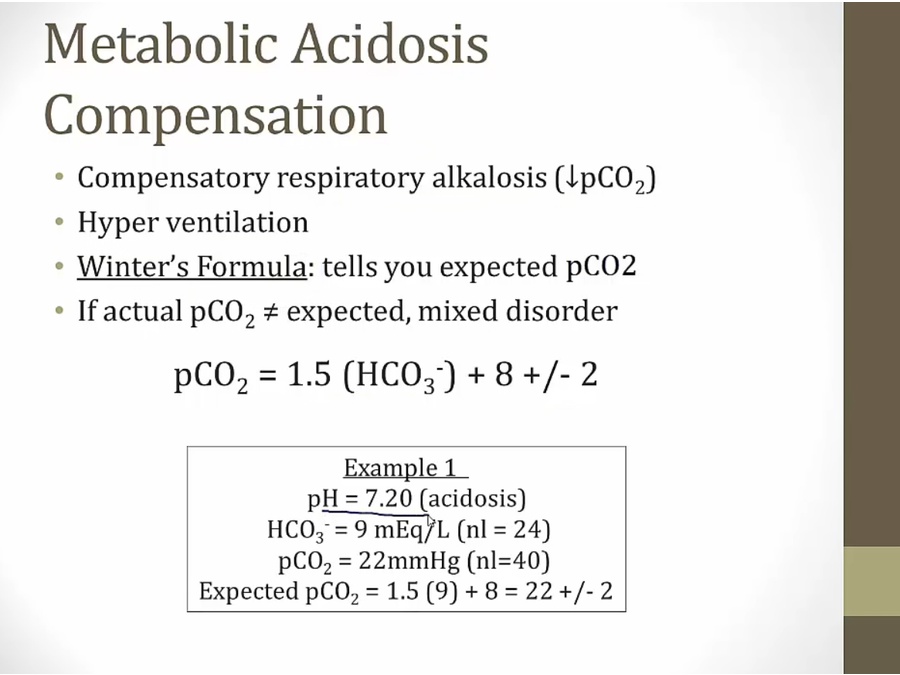
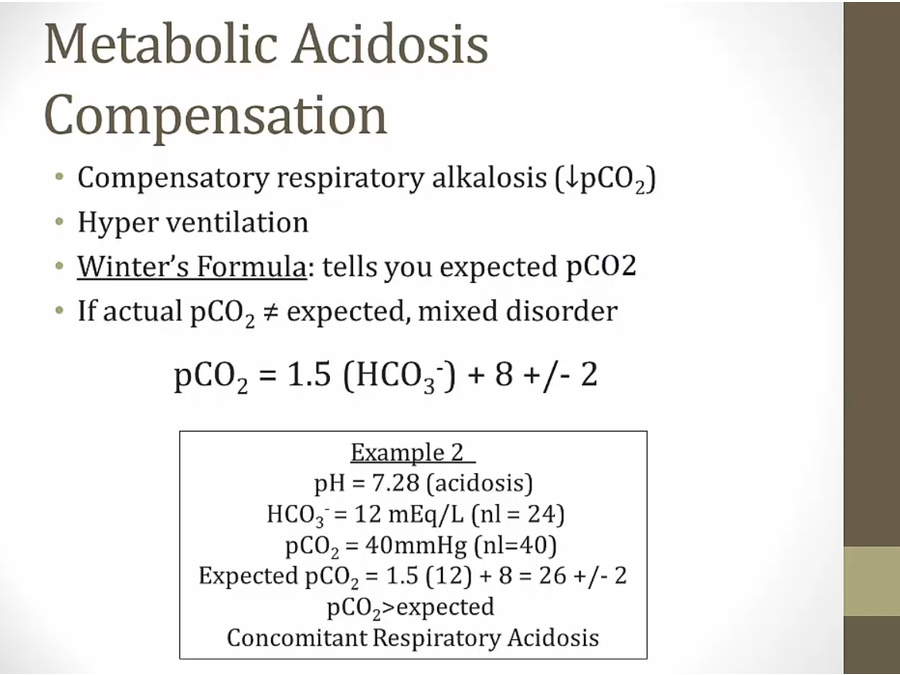
Metabolic Acidosis
Metabolic Alkalosis
Respiratory acidosis

Respiratory alkalosis

Last updated