09 Nephrology 2
ARF or AKI
Acute renal failure (ARF), also called acute kidney injury (AKI), is defined as an acute worsening in renal function with an increase in BUN and/or serum creatinine (SCr).
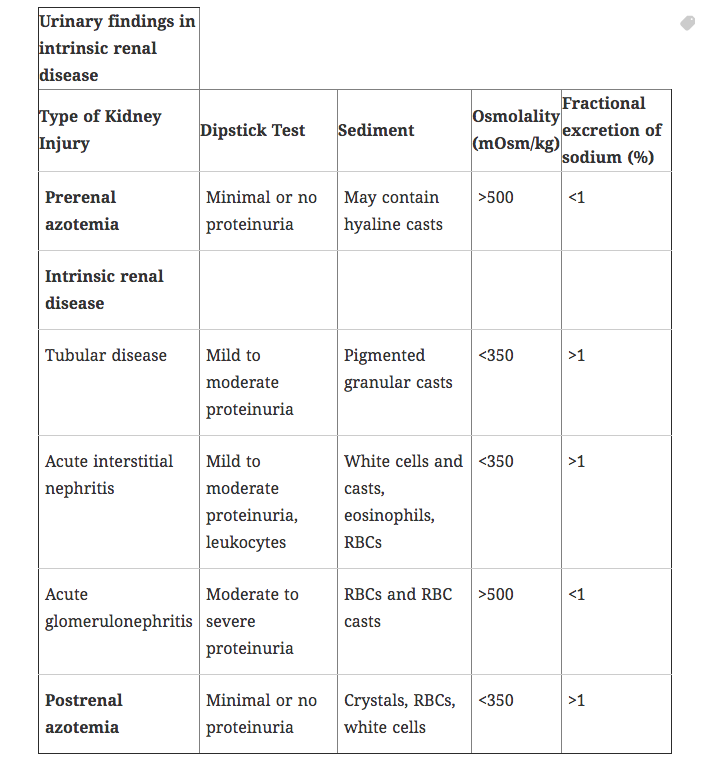
There are many causes of AKI, each divided into one of three categories: prerenal azotemia, intrinsic renal disease, and postrenal obstruction.
Prerenal
Pre-renal azotemia results from decreased renal blood flow and/or a decrease in glomerular hydrostatic pressure, leading to a decrease in the amount of nitrogenous waste products that are filtered.
Causes of pre-renal azotemia include any process that decreases renal blood flow:
Hypovolemia
Hypotension
Renal Artery Stenosis/Fibromuscular Dysplasia (FMH)
Decreased cardiac output
Medications that interfere with glomerular filtration (ACEIs, NSAIDs)
Elderly patients can be especially susceptible to volume depletion due to an impaired thirst response and often an inability to obtain food and water without assistance (eg, due to dementia).
By definition, the renal parenchyma is undamaged. This is key in differentiating pre-renal from intra-renal disease, in that because the parenchyma is unharmed, concentration ability is preserved.
Pre-renal azotemia that leads to renal damage (e.g. due to hypoxia) is considered to have progressed to intrinsic renal disease.
Intrinsic
Intrinsic renal disease (intra-renal) is defined as damage to the actual renal parenchyma that results in elevated levels of nitrogenous wastes.
There are three basic categories of intrinsic kidney disease based on the location of the disease: Glomerular disease, tubular-interstitial disease, and vascular disease.
Glomerular disease can result from disease to the glomerulous and/or small blood vessels. Acute glomerulonephritis is often a result of rapidly progressive glomerulonephritis (RPGN):
Type I RPGN: Goodpasture syndrome
Type II RPGN: Poststreptococcal glomerulonephritis, Lupus nephritis, IgA nephropathy
Type III RPGN: Wegener granulomatosis
Tubular-interstitial disease is often caused by:
Acute Tubular Necrosis (ATN): Can be caused by ischemic or nephrotoxic insult. Ischemic ATN is the most common cause of AKI. Microscopic examination of urine reveals epithelial casts which have degenerated to form pigmented, muddy-brown renal tubular casts in urine.
Acute Interstitial Nephritis
Vascular diseases of the kidney include:
Intrarenal vascular occlusion - renal artery/vein thrombosis, thrombotic microangiopathies: HUS (hemolytic uremic syndrome), TTP (thrombotic thrombocytopenic purpura)
Intrarenal vasculitis - Wegener granulomatosis
Postrenal
Post-renal azotemia (postrenal AKI) results from obstruction of urine outflow. The kidneys are intrinsically normal.
Obstruction of urethra by BPH (benign prostatic hyperplasia) is the most common cause of postrenal AKI.
Other causes of post-renal AKI include:
Nephrolithiasis (kidney stones in urethra or impacted at bladder neck)
Obstruction due to neoplastic invasion/extension (e.g. neoplasia of cervix, prostate, bladder)
Bilateral obstruction of ureters (e.g. retroperitoneal fibrosis—ureters are retroperitoneal structures)
Bilateral obstruction of kidneys (e.g. bilateral staghorn stones)
Patients often present with complaints related to the underlying cause of the acute kidney injury. Possible symptoms include:
Fatigue
Anorexia
Nausea
Oliguria
Hematuria
Flank pain
Altered mental status
NOTE: The most common symptoms of AKI are weight gain and edema due to a positive water and sodium balance.
A history, physical, and lab findings are critical to finding the underlying cause of acute kidney injury (e.g. dehydration, medication use, urinary symptoms). It is important to note that AKI may present as oliguric, anuric or nonoliguric.
Diagnosis
Imaging can help diagnose the underlying pathology (e.g. stones, BPH, vascular disease):
Ultrasonography (kidney size/hydronephrosis, hydroureter)
CT Scan (nephrolithiasis)
Renal arteriography (renal artery occlusion)
Renal biopsy (if suspicion for glomerulonephritis or AIN)
The treatment of acute kidney failure mainly depends on the underlying condition. Some general treatment measures include the following:
Prevention (avoid nephrotoxic agents or those that decrease renal blood flow)
Correct underlying fluid imbalance (IVF v. diuresis)
Correct underlying electrolyte imbalance (i.e. hyperkalemia, hypocalcemia)
The patient may need dialysis if uremia is prolonged and symptomatic or if electrolyte/acid-base imbalances are refractive to therapy
Treatment
Treatment for pre-renal AKI mainly focuses on maintenance of euvolemia and treatment of the underlying disorder. A swan-ganz catheter may be placed if the patient is unstable.
Treatment for intra-renal AKI is mostly supportive, especially once acute tubular necrosis has developed. Other helpful methods include elimination of offending agents and diuresis if the patient is oliguric.
Immunosuppressive medications may be helpful if the cause is determined to be related to glomerulonephropathy or vasculitides.
Postrenal AKI is sometimes treated with bladder catheterization or surgical removal of the obstructing pathology.
The prognosis in acute kidney injury (AKI) is generally good, with more than 80% of patients recovering completely.
In general, the prognosis decreases with increasing age and severity of AKI.
The most common cause of mortality in AKI is infection, accounting for up to 75% of deaths. The second most common cause involves cardiorespiratory complications.
ATN
There are 2 major categories of acute tubular necrosis (ATN): Ischemic and nephrotoxic. Regardless of etiology, the pathophysiology of ATN involves:
Disturbances in renal blood flow
Tubular injury
Ischemia
For example, consider the pathophysiology of ischemic ATN—renal ischemia causes:
Intrarenal vasoconstriction (net effect = afferent arteriolar vasoconstriction) → ↓ GFR → oliguria
Damage of tubules in outer renal medulla, especially the straight portion of proximal tubule and thick ascending limb of Henle’s loop (the two parts of the nephron that are most susceptible to hypoxic injury due to high ATP demand) → renal tubular cell necrosis and detachment from the basement membrane → luminal obstruction by pigmented renal tubular cell casts (Tamm-Horsfall protein + entrapped cells) → ↑ intratubular pressure → ↓ GFR → oliguria.
Ischemia causes intrarenal vasoconstriction (net effect = afferent arteriolar vasoconstriction) by 2 different mechanisms:
Ischemia → loss of tubule cell polarity → redistribution of basolateral Na-K-ATPase to luminal tubular cell surface → ↑ Na delivery to distal tubule → intrarenal vasoconstriction via a paracrine mechanism
Ischemia → endothelial damage → ↑ vasoconstrictors (endothelin) (vasoconstrictor) and ↓ vasodilators (NO, PGI2) → intrarenal vasoconstriction
Ischemic ATN is most commonly caused by pre-renal failure, resulting from anything that compromises renal perfusion, including:
1. Effective circulating blood volume (preload):
Hypovolemia—eg, vomiting, diarrhea, burns, hemorrhage, dehydration, diuretic overuse
Systemic vasodilation—eg, septic shock
Cirrhosis (↓ albumin production → ↓ colloid oncotic pressure → → ↓ intravascular volume)
2. ↓ Cardiac output—e.g. CHF (congestive heart failure), cardiogenic shock
3. NSAIDs (↓ PGI2 → ↓ vasodilation of afferent arteriole → ↓ GFR) or ACE inhibitors (↓ angiotensin II → ↓ vasoconstriction of efferent arteriole → ↓ GFR) can precipitate prerenal failure in patients who already have poor renal perfusion.
Nephrotoxic
Aminoglycosides are the #1 cause of nephrotoxic ATN. Other etiologies include:
Amphotericin B
Cisplatinum
Heavy metals: lead, mercury
Radiographic contrast media
Gram negative sepsis
Myoglobulinuria as a result of trauma / crush injuries or intense exercise (exercise-induced myoglobinuria)
Mechanism of nephrotoxic ATN:
Tubular toxicity
Myoglobin precipitation and tubular obstruction
Direct injury to proximal convoluted tubule from gentamicin, mercuric chloride or ethylene glycol (antifreeze). Note, most other drugs cause an interstitial nephritis.
In ethylene glycol acute tubular necrosis one would see massive intratubular oxalate crystal deposits with a polarized light.
Phases
Regardless of etiology (ischemic vs. nephrotoxic), ATN is characterized by 3 phases:
Initiation phase
Maintenance (oliguric) phase
Recovery (polyuric) phase
Initiation phase (first 36 hours):
Inciting ischemic event or nephrotoxin exposure → slight ↓ in urine output with an ↑ in BUN.
Maintenance (oliguric) phase:
Sustained oliguria (40-400mL/day).
↑ ECF (extracellular) volume → can be associated with weight gain, edema, pulmonary vascular congestion.
Hyperkalemia may or may not be present. → EKG changes (peaked T waves, depressed ST segment, prolonged PR interval, wide QRS complex), heart block, arrhythmias (eg, ventricular fibrillation) → ↑ risk of sudden cardiac death.
Retention of H+ and unmeasured anions (sulfate, phosphate, urate) → ↑ anion gap metabolic acidosis.
Recovery (polyuric) phase (2-3 weeks after inciting event if patients survive the oliguric maintenance phase):
Brisk diuresis (up to 3L/day) → urinary loss of K+, Ca2+, Mg2+, PO43- (because the renal tubules are still damaged at this point).
Hypokalemia (one of most serious complications of recovery phase of ATN) → EKG changes (flattening or inversion of T waves, U waves, depressed ST segment), premature atrial/ventricular contractions, arrhythmias (eg, atrial fibrillation).
Renal tubular function (concentrating ability) eventually recovers → BUN and serum Cr return to baseline.
The diagnosis of acute tubular necrosis is often one of exclusion of other causes of acute renal failure (ARF); in the majority of cases of ATN there is often a predisposing factor (e.g. contrast dye, aminoglycoside antibiotics, and/or rhabdomyolysis).
Urinalysis may show muddy brown granular casts (very common presentation) or be benign. The fractional excretion of sodium in most forms of ATN > 3. Exceptions can include contrast and rhabdomyolysis: the FeNa may be less than 1 in these disorders (due to intense renal vasoconstriction).
Treatment
The treatment of acute tubular necrosis is often supportive and often depends on identifying the underlying cause and treating it (e.g. fluids for rhabdoymolysis).
Avoid use of nephrotoxic medications if at all possible.
If at all possible avoid the use of dye in someone with CKD to reduce the risk of dye-induced nephropathy.
If the kidney function continues to worsen, dialysis may be needed.
During the diuretic phase of ATN, monitor electrolyte levels and volume status closely.
Acute tubular necrosis is the most common cause of intrarenal acute kidney injury (AKI). Recall that AKI is defined as an abrupt ↑ in serum creatinine or an abrupt ↓ in urine output.
AIN
Acute interstitial nephritis is an acute inflammation of the renal interstitium (edema + prominent mononuclear and eosinophilic infiltrate) that resolves following treatment of the underlying cause.b
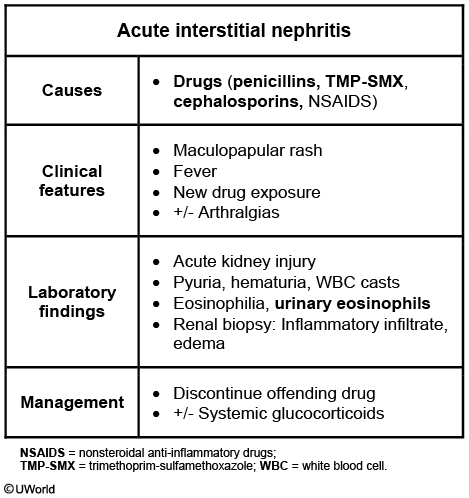
Interstitial nephritis is seen with the use of many drugs, remembered with the mnemonic "Please Note All Drugs that Can Possibly Scar Renals”
Penicillin derivatives—eg, methicillin, ampicillin
NSAIDs
Allopurinol
Sulfa-derived Diuretics—eg, thiazides, furosemide, acetazolamide
Cephalosporins
Proton pump inhibitors
Sulfonamide antibiotics—sulfamethoxazole, sulfisoxazole
Sulfasalazine—used to treat Crohn disease, ulcerative colitis, rheumatoid arthritis
Rifampin—RNA polymerase inhibitor used mainly to treat TB
Heavy metals and toxins can cause interstitial nephritis:
Cadmium
Lead
Copper
Mercury
Toxins from some mushrooms
Interstitial nephritis can also be caused by systemic diseases and pathologies:
Infections (Streptococcus spp., Legionella pneumophila)
Sarcoidosis
Amyloidosis
SLE
Myoglobinuria
High uric acid levels
Acute interstitial nephritis presents acutely with symptoms of acute renal failure, as well as:
Fever
Rash
Nausea and vomiting
Malaise
Acute interstitial nephritis will result in an increase in creatinine and eosinophilia.
Urinalysis will often show granular and epithelial casts and wbc casts from the shedding of renal tubular cells.
A biopsy of the kidneys will show infiltration of inflammatory cells throughout the interstitium as well as tubular cell necrosis.
There are many serious complications of interstitial nephritis, including:
Acute tubular necrosis
Acute or chronic renal failure
Renal papillary necrosis
In addition to removing/treating any underlying cause, treatment of acute interstitial nephritis is mostly supportive.
If severe enough, corticosteroids can also be useful.
CKD
Chronic kidney disease is defined as either decreased kidney function (GFR <60 mL/min) or kidney damage (urinary albumin excretion of ≥30 mg/day) for ≥3 months, regardless of the etiology.
The most common causes of chronic kidney disease are:
Type II DM (most common)
Hypertension
Chronic glomerulonephritis
Stage I CKD refers to a GFR ≥ 90 mL/min. There is normal kidney function but urine, structural or genetic findings suggest kidney disease.
Stage II CKD refers to a GFR 60-89 mL/min. Mildly reduced kidney function.
Stage III CKD refers to a GFR of 30-59 mL/min. Moderately reduced kidney function.
Stage IV CKD refers to a GFR of 15-29 mL/min. Severely reduced kidney function.
Stage V CKD is GFR < 15 mL/min. Uremic symptoms can be present at this stage. Very severe, or end-stage kidney failure.
There are many manifestations of chronic kidney disease. They include:
Altered mental status
Hypertension
Nausea & vomiting
GI bleeding
Peripheral neuropathy
Lab values indicative of chronic kidney disease include:
Elevated BUN & Cr
Increased K+ & phosphate
Decreased Na+ & Ca2+
Normochromic, normocytic anemia
Metabolic acidosis
Small kidneys on ultrasonography suggest advanced stage chronic kidney disease (CKD) that is unlikely to recover.
Note that the presence of normal-sized kidneys on ultrasonography does not rule out CKD.
Retention of sodium and water contributes to hypertension and accelerated atherosclerosis, peripheral edema, and congestive heart failure.
Advanced CKD can be associated with an anion-gap metabolic acidosis.
There is decreased excretion of hydrogen ions (H+) in the form of ammonium, which maintains the metabolic acidosis.
Decreased renal excretion of organic anions such as sulfate, phosphate, urate and hippurate increase the anion gap and also maintain the metabolic acidosis (i.e. sulfur in the form of sulfuric acid, phosphorus as phosphoric acid and so forth).
The cause of anemia in CKD can be multifactorial. The leading cause of anemia in CKD is decreased erythropoietin production which causes a normochromic, normocytic anemia. There are other contributing factors to anemia in CKD such as:
Anemia due to inflammation
Blood loss due to increased bleeding
Marrow fibrosis due to renal osteodystrophy
Renal osteodystrophy is a complex interplay between calcium, phosphorous, parathyroid hormone, and Vitamin D. There are several contributing factors to the characteristic bone changes in CKD.
Hyperphosphatemia can stimulate PTH secretion from the parathyroid gland.
Decreased production of 1-α-hydroxylase also contributes to increased PTH secretion (ie secondary hyperparathyroidism).
Hypocalcemia is due to decreased absorption from the GI tract. Calcium can also precipitate with phosphorous out into tissues (metastatic calcification).
Osteitis fibrosa cystica (AKA “brown tumors”) is the most common type of renal osteodystrophy caused by a high rate of bone turnover. The intact PTH levels are often very elevated.
There are several mechanisms of hyperkalemia in kidney disease.
Advanced kidney disease: inability of kidneys to excrete a potassium load. Decreased renal excretion of K leads to hyperkalemia.
Medications such as ACE inhibitors, Digoxin and NSAIDs can worsen kidney function and be a cause of hyperkalemia in someone with CKD.
Hyperkalemia can also be aggravated by insulin deficiency (recall that insulin causes the trans-cellular shift of K → into cells).
The increased bleeding risk in advanced CKD is due to uremia-induced platelet dysfunction.
The risk of infection is increased in advanced CKD due to uremia induced-neutrophil dysfunction.
Treatment
Reducing proteinuria in Stage I CKD is vital.
ACE Inhibitors and/or Angiotensin receptor blockers (ARBs) can reduce proteinuria and preserve kidney function
For diabetes, obtain albumin/creatinine ratios on annual basis. Tight glucose control can prevent worsening of kidney disease and proteinuria
If edema is present, the use of a loop diuretic may be added
Erythropoietin stimulating agents (ESA) are used in the treatment of anemia of chronic disease in patients with CKD.
Since iron deficiency is common in patients with CKD, iron status should be evaluated prior to initiating treatment with an ESA (e.g. erythropoietin). Patients with inadequate iron levels should be given supplemental iron.
Failure to correct iron deficiency prior to starting an ESA (e.g. erythropoietin) can result in a microcytic, hypochromic anemia.
Adverse effects associated with recombinant EPO include:
Hypertension (most common)
Headache
Flu-like syndrome
Red cell aplasia (rare, due to anti-EPO antibodies)
Dietary modifications in the treatment of chronic kidney disease include:
Low phosphorous diet and the use of phosphorous binders are used for hyperphosphatemia
Secondary hyperparathyroidism is treated by lowering phosphorous levels and normalizing calcium levels. The use of vitamin D analogs (a synthetic 1-alpha hydroxylase) can also be used to lower PTH levels
Hyperkalemia is treated with a low potassium diet. The use of sodium bicarbonate and diuretics as needed. If hyperkalemia is refractory, dialysis may be needed.
Protein restriction is utilized in advanced CKD to preserve kidney function and prevent overworking of the kidney
Sodium bicarbonate can be used to treat metabolic acidosis. Treating acidosis in CKD can prevent progression of CKD.
Loop diuretics can be used for volume management in CKD.
Absolute indications for emergent dialysis can be remembered with the mnemonic AEIOU:
Acidosis
Electrolyte abnormalities
Ingestion of toxins
Overload of fluid
Uremia
Diuretics
There are several classes of diuretic medication including:
Thiazide diuretics
Loop diuretics
Potassium-sparing diuretics
Carbonic anhydrase inhibitors
Mannitol
Thiazide
Thiazide diuretics (e.g. hydrochlorothiazide, chlorthalidone) inhibit the NaCl co-transporter in the distal convoluted tubule.
First drug of choice in most newly-diagnosed essential hypertension; also useful in patients with edema, congestive heart failure, nephrogenic diabetes insipidus and osteoporosis.
Thiazide toxicities include:
Decreased serum levels of:
K+ (hypokalemia) – check potassium levels regularly
Na+ (hyponatremia)
H+ (metabolic alkalosis)
Increased serum levels of (mnemonic ‘GLUC’):
Glucose (hyperglycemia)
Lipids (hyperlipidemia)
Uric acid (hyperuricemia)
Calcium (hypercalcemia)
Loop
Loop diuretics (i.e. furosemide, bumetanide, ethacrynic acid) block the NKCC (Na+/K+/2Cl-) symporter in the thick ascending limb of the Loop of Henle.
Loop diuretics are particularly useful in clinical settings of volume overload including congestive heart failure and edema.
Loop diuretic toxicities include (mnemonic ‘OHHH DANG’):
Ototoxicity
Hypokalemia
Hypocalcemia
Hypomagnesemia
Dehydration
Allergy (sulfa – except ethacrynic acid)
Nephritis (interstitial)
Gout
K sparing
Potassium-sparing diuretics (i.e. amiloride, triameterene, spironolactone and eplerenone) act at the collecting tubule of the nephron.
Potassium-sparing diuretics are particularly useful as adjuvants to other diuretic medications, especially if hypokalemia is a concern.
Because spironolactone and eplerenone are competitive inhibitors of aldosterone receptors, they are only active in the presence of aldosterone.
Toxicities of spironolactone include:
Hyperkalemia
Gynecomastia
Amenorrhea
Other anti-androgen effects
CA Inhibitors
Carbonic anhydrase inhibitors (e.g. acetazolamide) act at the proximal convoluted tubule by inhibiting the production and reabsorption of filtered bicarbonate.
Carbonic anhydrase inhibitors are rarely used in the treatment of hypertension and more often used in the treatment of intracranial hypertension.
Other conditions treated with acetazolamide include altitude sickness, increased intra-ocular pressure and alkalinization of urine for the treatment of renal calculi.
Toxicities of acetazolamide include:
Hyperchloremic metabolic acidosis
Hypokalemia
Ammonia toxicity (due to decreased clearance)
Neuropathy
Sulfa allergy
Mannitol is an osmotic diuretic that increases tubular fluid osmolarity, thereby increasing urine flow.
Mannitol is most often used in treatment of increased intracranial pressure.
Mannitol toxicity includes pulmonary edema and intravascular dehydration.
Metaboic Acidosis
Metabolic acidosis is defined as an acidotic pH (< 7.37) with a decrease in HCO3- levels.
In metabolic acidosis, look for the following general values:
pH
[H+]
[HCO3-]
PCO2
Compensation
low
high
very low
low
Hyperventilation
Causes of metabolic acidosis are separated into two categories: increased anion gap and normal anion gap.
Anion gap calculation:
AG (mEq/L) = [Na+] – ([Cl-] + [HCO3-])
Normal = 10-15 mEq/L
Increased AG
Causes of anion gap metabolic acidosis include:
Ketoacidosis (starvation, DKA, alcohol use)
Exogenous toxins (methanol, ethylene glycol, salicylates)
Lactic acidosis (ischemia, shock)
Renal failure (decreased NH4+ excretion)
Significant uremia
Drugs: paraldehyde, isoniazid (INH)
Useful mnemonic: MUDPILES (Methanol, Uremia, DKA, Paraldehyde,INH, Lactic acidosis, Ethylene glycol, Salicylates)
An increased osmolol gap is suggestive of toxic alcohol ingestion as a cause of the metabolic acidosis.
The osmolar gap should be calculated in selected cases of increased anion gap acidosis, such as when ethanol, methanol or ethylene glycol toxicity is suspected. It is calculated by determining the difference between the measured and calculated serum osmolality.
Calculate the osmolol gap (OG) as follows:
$[Osm]_{measured}-(2[Na]+\frac{[glucose]}{18}+\frac{[Bun]}{2.8})$
[Osm]measured is the measured serum osmolality, and [Na], [glucose], and [BUN] are the measured concentrations of sodium, glucose, and BUN, respectively.
A normal OG is < 10 mOsm/kg.
Normal AG
Normal anion gap metabolic acidosis (hyperchloremic metabolic acidosis) can be further divided into 4 categories (low aldosterone):
GI HCO3- loss
renal acidosis
drug-induced hyperkalemia with renal insufficiency
and other causes
Normal anion gap due to GI HCO3- loss is seen in:
Diarrhea (most common)
External pancreatic or small-bowel drainage
Jejunal and ileal loops
Normal anion gap hypokalemic renal acidosis is seen in:
Type 1 Renal Tubular Acidosis (RTA) - Failure of distal tubule cells to secrete H+ and generate new HCO3-.
Drugs: Ifosfamide, Amphotericin B
Type 2 RTA - Decreased HCO3- reabsorption in the proximal convoluted tubule
Drugs: acetazolamide, topiramate
Normal anion gap hyperkalemic renal acidosis is seen in Type 4 RTA (hypoaldosteronism).
Drug-induced hyperkalemia with renal insufficiency leading to normal anion gap metabolic acidosis is seen in:
Potassium-sparing diuretics (amiloride, triamterene, spironolactone)
Trimethoprim
ACEIs and ARBs
NSAIDs
Cyclosporine
Other causes of normal anion gap metabolic acidosis include:
Acid loads (ammonium chloride)
Expansion acidosis from rapid saline administration
Hippurate
If a patient has a normal anion gap, GI HCO3- loss can be differentiated from RTAs as potential causes by calculating the urine anion gap (UAG). The UAG is the difference between measured anions and cations in the urine: UAG = [Na]urine + [K]urine – [Cl-]urine
Since the major unmeasured urinary cation is NH4, a negative UAG suggests high levels of NH4 excretion. This is an appropriate response, suggesting normal renal function and therefore GI loss of HCO3-.
Conversely, a positive UAG suggests low NH4 excretion, indicating renal tubular dysfunction.
Compensation for metabolic acidosis can be made via the respiratory system by hyperventilation, causing a reduction in PCO2.
Respiratory compensation for metabolic acidosis is triggered by a drop in arterial pH, resulting in stimulation of central and peripheral chemoreceptors controlling respiration.
To estimate the expected PCO2 range based on respiratory compensation, the Winter’s formula can be used. The Winter’s formula is calculated as:
Expected PaCO2 = 1.5 (measured HCO3-) + 8 +/- 2
If the PCO2 falls within the acceptable range, the patient has a simple metabolic acidosis with appropriate respiratory compensation.
If the PCO2 does not measure within acceptable range, there is likely another acid-base disorder present.
Treatment
The treatment of normal anion gap metabolic acidosis should focus on correcting the underlying pathology.
Seizures: the most appropriate management of this patient is observation and a repeat chemistry panel after approximately 2 hours. Norepinephrine acts as a positive inotrope and vasoconstrictor and is often used in patients with lactic acidosis due to hypotension and poor organ perfusion (eg, sepsis) that persists following fluid resuscitation.
Should the acid-base disturbance be acute and severe, treatment involves administration of NaHCO3.
It is generally recommended in patients with severe acute metabolic acidosis with pH less than 7.1. Administration of sodium bicarbonate may cause myocardial depression and increased lactic acid production; therefore, in patients with pH >7.1
Metabolic Alkalosis
Metabolic alkalosis is defined as a serum pH > 7.43 with an increase in serum HCO3- levels.
Metabolic alkalosis can be due to a gain in HCO3- or, more commonly, excessive loss of H+.
H+ loss often results from the loss of H+ rich GI fluids (e.g. vomiting).
Contraction alkalosis is volume loss around a fixed amount of HCO3-. Though the total body HCO3- levels have not changed, the concentration has and therefore the pH increases.
The kidneys have the ability to excrete very large amounts of HCO3-, so in order to maintain metabolic alkalosis there often must be impaired renal function. This can result from:
Decreased GFR
Chloride depletion
Volume contraction
Hypokalemia
The ability to secrete HCO3- in the kidneys depends on the countertransport of Cl-. In hypochloremia, the decrease in filtered Cl- is sensed by the macula densa, resulting in a decrease in filtered HCO3- and increased aldosterone release, both of which worsen the alkalosis. Cases of metabolic alkalosis are often described as chloride responsive or chloride unresponsive (see below).
In metabolic alkalosis, expect to see the following values:
pH
[H+]
[HCO3-]
PCO2
Compensation
High
Low
Very High
High
Hypoventilation
Urine [Cl-] < 10 mEq/L suggests chloride responsive metabolic alkalosis as well as volume depletion, and urine [Cl-] > 20 mEq/L suggests chloride unresponsive metabolic alkalosis.
Urine [K+] is normally low in metabolic alkalosis.
Signs and symptoms of metabolic alkalosis are similar to those of hypocalcemia:
Confusion
Paresthesias
Tetany
Muscle cramping
Seizures
Arrhythmias
There are also often signs and symptoms of hypovolemia, since volume contraction results in many causes of metabolic alkalosis (vomiting, diuretic use).
Treatment
Chloride responsive metabolic alkalosis is treated with saline, which results in increased filtered chloride and increased HCO3- secretion.
Chloride unresponsive metabolic alkalosis does not respond to saline.
Hypokalemia potentiates alkalosis of any cause. Correcting the hypokalemia is necessary to adequately treat the alkalosis.
Acetazolamide is used if saline administration and correcting the hypokalemia fails to resolve the metabolic alkalosis.
If alkalemia is severe (pH > 7.70) with hypervolemia, HCl can be administered through a central line.
Pyelonephritis
Pyelonephritis is an infection of the kidneys. The most common infectious agent is E. coli.
Less common pathogens include:
Klebsiella
S. saprophyticus
Proteus
Candida (in immunocompromised patients)
Pyelonephritis generally occurs from an ascending infection, beginning in the lower urinary tract.
Risk factors for the development of pyelonephritis include:
Urinary tract obstruction
Frequent sexual intercourse
A new sexual partner
Spermicide use
Previous history of pyelonephritis
Immunocompromised status
Symptoms consistent with pyelonephritis include:
Flank pain
Costovertebral tenderness
Urinary symptoms (frequency, dysuria, urgency)
Chills
Nausea,vomiting
Fever
On urine microscopy, pyelonephritis will cause white blood cell casts and 10^5 bacteria/mL of urine.
Other lab findings include elevated WBCs, ESR, and C-reactive protein.
Pregnant women with pyelonephritis are at an increased risk of preterm labor and delivering a low birth weight baby.
Treatment
For less severe/uncomplicated cases of pyelonephritis, the treatment is 1-2 days of IV fluoroquinolones, aminoglycosides, or 3rd generation cephalosporins, followed by oral antibiotics.
For cases of complicated pyelonephritis (i.e. in pregnancy, diabetes, obstruction, renal failure, etc.), patients may require 2-3 weeks of IV antibiotics.
DI
Diabetes insipidus (DI) is a leading cause of euvolemic hypernatremia. It typically presents with severe polyuria and mild hypernatremia. It can be divided into two types based on urine osmolality, as well as etiology.
Based on urine osmolality, DI may be complete or partial.
Complete DI - the urine osmolality is less than 300 mOsm/kg (often less than 100 mOsm/kg)
Partial DI - urine osmolality ranges from 300-600 mOsm/kg.
The serum osmolality is elevated in both types.
Based on etiology, DI may be central or nephrogenic.
Central DI is due to decreased production of antidiuretic hormone (ADH). Common causes include trauma, hemorrhage, infection, and tumors.
Nephrogenic DI results from renal ADH resistance. Common causes include hypercalcemia, severe hypokalemia, tubulointerstitial renal disease, and medications. The most commonly implicated mediations are lithium, demeclocycline, foscarnet, cidofovir, and amphotericin.
Treatment
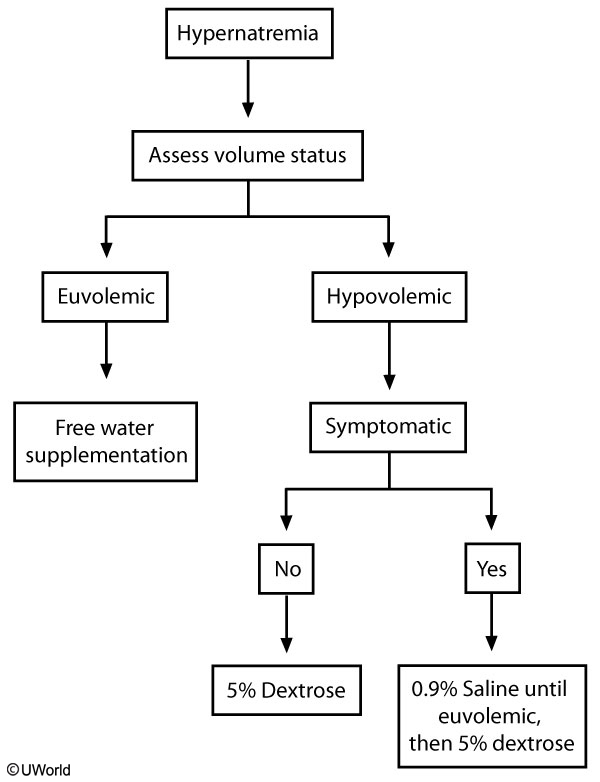
The first step is to restore volume with isotonic fluids (0.9% saline). Isotonic fluid is not usually used in hypernatremia, but it is recommended in patients with marked volume depletion and hemodynamic instability. Once the patient is euvolemic, the fluid can be switched to a hypotonic fluid (5% dextrose preferred over 0.45% saline) for free water supplementation. The serum sodium should be corrected by 0.5 mEq/dL/hr without exceeding 12 mEq/dL/24 hr. Cerebral edema can occur if the sodium is corrected too quickly.
Last updated