06 GI
Stomach
PUD
Peptic ulcer disease (PUD) refers to a defect within the mucosal wall of the duodenum and stomach that typically results in recurrent episodes of epigastric pain.
The duodenum and stomach both contain a mucosal lining that protects the underlying cells from its acidic environment. Epithelial cells line these structures and secrete bicarbonate to help buffer the acid to prevent mucosal damage. Peptic ulcer disease results when these protective barriers are disrupted.
Peptic ulcer disease may be caused by any of the following:
H. pylori infection
Drugs (NSAIDs, SSRI)
Alcohol
S moking
S evere physiologic stress (burns, head trauma, surgery)
Hypersecretion of acid
H. pylori infection and NSAID use are the two most common causes of peptic ulcer disease. H. pylori is a gram-negative bacteria that releases enzymes which increase acid secretion and inhibit bicarbonate production within the stomach, resulting in inflammation and ulceration.
Patients with peptic ulcer disease typically present with recurrent episodes of epigastric pain which they describe as a dull, gnawing, burning sensation.
The pain associated with peptic ulcer disease is often worsened by eating in gastric ulcers, but relieved with eating in duodenal ulcers. Weight gain can occur with duodenal ulcers since their pain improves with eating, while weight loss is typically seen in gastric ulcers due to increased pain with food.
Other possible manifestations of peptic ulcer disease include:
Belching
Bloating
Heartburn
Nausea and vomiting
There are some symptoms however that require immediate gastroenterology referral, known as alarm symptoms:
Unexplained weight loss
Early satiety
Hematemesis (vomiting blood)
Melena (dark, tarry stools)
Hematochezia (bloody stools)
Family history of gastrointestinal cancer
Painful or difficulty swallowing
Diagnosis
Upper endoscopy is the diagnostic test of choice in evaluating patients with peptic ulcer disease. Endoscopy allows for the direct visualization of gastric and duodenal ulcers, biopsies to rule out malignant lesions and allows for the detection of H. pylori infections.
H. pylori testing should be performed on all patients suspected of having PUD. There are many ways in which H. pylori can be tested:
Mucosal biopsy (most accurate)
Rapid urease testing
Urease breath test
Fecal H. pylori antigen testing
H. pylori serum antibody testing. This test is not able to differentiate between past and active infections and therefore has minimal diagnostic utility.
Radiographic studies are usually not helpful in diagnosing PUD, unless a perforation has occurred which would show free air below the diaphragm on a chest x-ray.
PUD may result in complications such as:
Bleeding (hematemesis, melena, hematochezia)
Perforation
Death
The most common complication from peptic ulcer disease is hemorrhage, not perforation. Bleeding results when ulcers erode the vasculature within the walls of the gastric and duodenal mucosa.
Treatment
Treatment of PUD typically consists of:
Eradicating H. pylori infections with triple therapy antibiotics
Avoiding exacerbating factors (NSAIDS, alcohol, smoking)
Proton pump inhibitors (PPI)
Acute bleeding ulcers and perforation require surgical intervention.
First-line therapy for H. pylori infection includes:
Proton pump inhibitor (e.g. omeprazole, lansoprazole)
Amoxicillin
Clarithromycin
In penicillin allergic patients, the amoxicillin is replaced with metronidazole.
Bleeding ulcers require endoscopic intervention to locate the source, then they can either be ligated with a suture or cauterized to control the bleeding.
Surgery is indicated once an ulcer has perforated the bowel wall. Duodenal perforation is typically repaired with an omental (Graham) patch, where a piece of omentum is sutured over the perforation. Gastric perforation is repaired by partially resecting the damaged gastric wall--an omental patch may be used here as well.
Gastric Carcinoma
Gastric cancer is 90% adenocarcinoma, and is further divided into intestinal and diffuse subtypes.
Intestinal gastric adenocarcinoma is more common in older patients and has a better prognosis. It also has a higher association with H. pylori infection and chronic gastritis than the diffuse type.
Diffuse gastric adenocarcinoma is more common in younger and female patients, and is the most common subtype in the United States.
Risk factors include GAS MASH:
Gastrectomy in the past for gastric ulcer Anemia (pernicious) and Achlorhydria Smoking
Menetrier’s disease A blood group Smoked fish or other nitrates H. pylori
Patients often present with:
Decreased appetite
Weight loss
Abdominal discomfort or pain
Nausea
Vomiting
In patients with the sudden dermatologic onset of seborrheic keratoses, consider an underlying gastric malignancy. This paraneoplastic phenomenon is known as the Leser–Trélat syndrome.
Dysphagia may occur with obstruction of the gastroesophageal junction.
Diagnosis is confirmed by upper endoscopy with biopsy.
Fecal occult blood test is often positive, with or without a history of melena.
CT of the chest, abdomen, and pelvis (in women) should be performed to evaluate for metastatic spread or a primary tumor.
Helicobacter pylori testing should also be done.
The classic physical exam findings for metastatic disease are supraclavicular (Virchow’s node) or periumbilical nodes (Sister Mary Joseph’s node).
Many gastric cancers are found in their later stages when metastases have already occurred.
Gastric cancer often metastasizes to the ovaries (Krukenberg’s tumor). Rectal (Blumer’s shelf) metastases are palpable on digital rectal exam.
Resection with lymph node dissection is preferred, with chemotherapy and radiation except in cases of early disease.
Palliative radiation is used in non-resectable cases.
Gastritis
Gastritis is defined as an inflammation and erosion of the gastric lining. The mucosa lining the stomach helps protect it from offending agents and when the mucosa becomes injured or damaged, it is unable to protect itself from further injury.
Gastritis most commonly occurs with:
NSAIDs (aspirin, ibuprofen)
Alcohol abuse
H. pylori infections
Other risks include portal hypertension, severe stress (burns, trauma, sepsis), and autoimmune disease.
Patients with gastritis will typically complain of a gnawing or burning sensation within their epigastric region, which may or may not be accompanied by nausea and vomiting.
Upper endoscopy with mucosal biopsy is the diagnostic modality of choice for gastritis. Endoscopy allows direct visualization of mucosal damage and biopsy helps the diagnostician determine the etiology.
Stool antigen and urea breath test are used to help rule out H. pylori infection.
Imaging such as a double-contrast barium study can help determine the extent of gastric erosions.
Gastritis may result in:
Anemia
Hematemesis
Ulcers
Perforation
Strictures
Weight loss
Gastric cancer
Treatment of gastritis includes the discontinuation of offending agents (NSAIDs, aspirin, alcohol), and administration of a proton pump inhibitor (PPI). Consider triple therapy (PPI + amoxicillin + clarithromycin) if H. pylori is found to be the cause of gastritis.
Antacids
Antacids directly neutralize the stomach acidity.
Side effects of antacids may include:
Constipation
Nausea
Diarrhea
H2 antagonists block histamine receptors, preventing histamine mediated acid release from parietal cells.
Side effects of H2 blockers include:
Headache
Diarrhea
Thrombocytopenia (rarely)
Cimetidine can cause gynecomastia and impotence
PPIs block the parietal cell H+/K+ ATPase.
Omeprazole is an inhibitor of the cytochrome P-450 system.
PPIs may also cause:
Rebound hyperacidity
Enteric and respiratory infections
Bone fractures (due to decreased calcium absorption)
Acute interstitial nephritis
Nutritional deficiencies (due to decreased B12 and magnesium absorption)
Urea breath testing for the diagnosis of H. pylori in patients on PPIs may yield false-negative results since PPIs can suppress H. pylori. Thus, patients should discontinue PPIs for a minimum of 2 weeks prior to undergoing a urea breath test.
Lactose Intolerance
Lactose intolerance is a malabsorption syndrome resulting from a deficiency in lactase. This leads to the inability to absorb lactose from the diet. It may also be secondary to other disease processes such as Crohn's disease or overgrowth of intestinal flora.
Lactase is normally located in the brush border of the enterocytes of the intestinal villi.
Deficiency of lactase may be primary or secondary:
Primary deficiency is the most common, in which lactase production decreases over time.
Secondary deficiency results from injury to the small intestine, such as in Crohn's disease, small bowel surgery, or infection.
Congenital absence of lactase is genetically linked and is very rare.
Symptoms are a result of an increased osmotic gradient in the gut. Following consumption of dairy products, patients may present with:
Abdominal pain
Bloating
Flatulence
Osmotic diarrhea
Vomiting (particularly in adolescents)
Stools of patients with lactose intolerance are usually bulky, frothy, and watery.
Borborygmi (rumbling noises made by gas and fluid in the intestines) may be audible on physical examination and to the patient.
Diagnosis is confirmed by either a lactose absorption test (positive test will reveal minimal increase in serum glucose after ingesting lactose) or a lactose breath hydrogen test.
The mainstay of treatment is a lactose-free or lactose-restricted diet. Patients must receive adequate amounts of other nutrients such as vitamins, protein, and fat. Lactase enzyme replacement is an option that may benefit some patients.
Gastroenteritis
The most common presentation for viral gastroenteritis is:
Diffuse watery diarrhea
Mild fever
Malaise
Viral gastroenteritis may result in severe dehydration, hypotension, and syncope.
Coxsackie B infections may result in:
Severe dehydration
Sepsis
Myocarditis
Pericarditis
Aseptic meningitis
Viral gastroenteritis is usually self-limited and typically treated with supportive therapy (e.g. fluid and electrolyte replacement). Antiemetics (e.g. ondansetron) and antimotility agents (e.g. loperamide) may be incorporated if vomiting and diarrhea become severe.
Viral gastroenteritis is inflammation of the stomach and intestines (commonly called the "stomach flu"). Four viruses commonly implicated in viral gastroenteritis include:
Norovirus
Echovirus
Rotavirus
Coxsackie virus
Norovirus, previously referred to as the Norwalk virus, is the most common cause of viral gastroenteritis in adults. The virus is endemic in the winter months and most commonly spread via the fecal-oral route.
Infected people tend to contaminate food and other surfaces by improper hand washing. Outbreaks commonly occur in restaurants, military facilities, healthcare facilities, schools, and cruise ships.
ECHO (Enteric Cytopathic Human Orphan) viruses are members of the Enterovirus genus and are found within the gastrointestinal tract. The echovirus typically infects via the fecal-oral route and is a common cause of gastroenteritis, aseptic meningitis, and myocarditis in the summer and fall.
Coxsackie viruses (A and B) are members of the Enterovirus genus and are primarily transmitted via fecal-oral route and by respiratory droplets. Type A is responsible for herpangina, conjunctivitis, and hand-foot-and-mouth disease, while type B is responsible for gastrointestinal inflammation.
Rotavirus most severely affects children under the age of 5 and is a vaccine-preventable cause of illness. It typically strikes during the winter months (December-May), and is usually acquired in settings such as daycare.
Reverse transcriptase polymerase chain reaction (RT-PCR) is the most sensitive and specific diagnostic method for Norovirus and echo virus, but it is not typically required.
Testing for fecal leukocytes and fecal blood is important in ruling out invasive diarrheal infections.
The best diagnostic modality for Coxsackie B viral infections is viral culture. Samples are typically taken from stool and rectal swabs, but can also be isolated from the oropharynx.
Liver Disease
Budd-Chiari
Budd-Chiari syndrome (or hepatic vein thrombosis) is defined as any thrombotic or obstructive process, which inhibits the outflow of blood within the hepatic venous system.
Most patients with Budd-Chiari syndrome have some underlying thrombotic risk factor. Some of the most common risk factors include:
Myeloproliferative disease (e.g. polycythemia vera)
Usage of oral contraceptives
Pregnancy
Malignancy
Inherited hypercoagulable diseases
The most common presentation is:
Hepatosplenomegaly
Ascites
Abdominal pain
Renal impairment
As the disease progresses signs of liver failure (eg jaundice, encephalopathy) and portal hypertension (eg ascites, hematemesis) become more prominent.
Venography is the gold standard for diagnosis, but is typically only used when initial non-invasive diagnostic tests (e.g. ultrasound, CT, MRI) are negative and there is a high suspicion for the disease.
Budd-Chiari syndrome may result in long term complications such as:
Liver failure
Renal failure
Spontaneous bacterial peritonitis
Portal hypertension (e.g. hematemesis)
Note: A way to remember these are that they are similar findings to those seen in a patient with cirrhosis.
Treatment of Budd-Chiari syndrome depends on the clinical presentation of the patient and extent of thrombosis. The most common form of treatment includes anticoagulation therapy with heparin and warfarin along with venous stenting.
Diuretics such as spironolactone and furosemide are incorporated to help with ascites and fluid overload.
Transjugular intrahepatic portosystemic shunt (TIPS) is another procedure used to help decompress the liver when portal hypertension is present. Liver transplant is a last resort.
Autoimmune Hepatitis
Autoimmune hepatitis (AIH) is a chronic disease of unknown etiology, which is characterized by:
Circulating autoantibodies
Hepatocellular inflammation
Fibrosis
Risk factors for autoimmune hepatitis include:
Caucasian race of northern European ancestry
Female gender
Acute hepatitis A and B infections
Patients with HLA haplotypes DR3 and DR4
Symptoms
Symptoms can vary widely in patients with AIH, and may include:
Asymptomatic
Fulminant hepatic failure
RUQ pain
Jaundice
Fatigue
Hepatosplenomegaly
Ascites
Diagnosis
The diagnosis of autoimmune hepatitis is generally based upon characteristic clinical and serological features, but liver biopsy may be required to confirm the diagnosis.
Other helpful serological studies include:
Anti-smooth muscle antibody (ASMA)
Anti-nuclear antibody (ANA)
Anti-liver kidney microsomal antibody (anti-LKM)
IgG immunoglobulins
Serum aminotransferase (AST and ALT)
Bilirubin
Alkaline phosphatase
AIH may result in cirrhosis, hepatocellular carcinoma, and eventual fulminant hepatic failure.
Treatment
The best initial therapy for AIH is prednisone and azathioprine.
Liver function tests are performed weekly for the first 6-8 weeks, then every 2-3 months to monitor therapy response.
Ischemic Hepatitis
Ischemic hepatitis is caused by a decrease in blood flow leading to diffuse, hypoxic liver injury.
Risk factors for ischemic hepatitis include:
Shock
Congestive heart failure
Blood loss
Hepatic artery thrombosis
Portal vein thrombosis
Sickle cell anemia
Any cause of hemodynamic instability
Patients with ischemic hepatitis typically present with:
Signs of hemodynamic instability
Nausea
Vomiting
Right upper quadrant pain
Ischemic hepatitis is best diagnosed clinically by:
A sudden increase in liver enzymes (AST and ALT greater than 1000 IU/L)
Early, rapid rise of lactate dehydrogenase
Recent hypotensive event
Other end organ damage
Ischemic hepatitis is typically managed by treating the underlying cause of the hemodynamic instability.
If left untreated, ischemic hepatitis may cause:
Encephalopathy
Jaundice
Acute hepatic failure
Hepatopulmonary syndrome
Acetaminophen overdose
The most significant effect of acetaminophen overdose is hepatotoxicity. Acetaminophen is primarily metabolized into N-acetyl-p-benzoquinoneimine (NAPQI), a highly reactive, toxic intermediate. At toxic doses, the hepatic glutathione pathway is saturated, and NAPQI reacts with cysteine groups on hepatic macromolecules leading to oxidative injury and hepatocellular necrosis.
Patients with acute liver injury due to acetaminophen toxicity can present along a spectrum of severity:
Within 1 day: asymptomatic or mild nausea/vomiting
After 24 hours: abdominal pain, increasing severity of other symptoms
In the most severe cases, hepatotoxicity and nephrotoxicity precedes multiorgan failure and eventual death
Cerebral edema is a potential complication of ALF that may lead to coma and brain stem herniation, and is the most common cause of death.
Acetaminophen is the most common cause of acute liver failure in the United States.
Acute liver failure is defined as the development of hepatic injury in a patient without cirrhosis or preexisting liver disease. All 3 of the following criteria are required for the diagnosis of acute liver failure:
Elevated aminotransferases
Hepatic encephalopathy
Prolonged PT (INR ≥1.5)
The diagnosis of acetaminophen toxicity relies on the history as most patients are initially asymptomatic.
Acetaminophen levels can be checked to determine the extent of overdose.
Management
Both liver and kidney function should be monitored closely by checking:
Transaminases
Coagulation studies
BUN
Creatinine
The antidote to acetaminophen overdose, N-acetylcysteine (NAC), is believed to regenerate hepatic glutathione stores, which helps with the elimination of free radicals.
NAC is most effective when administered within the first 8 hours of overdose.
If patients present within 4 hours of acetaminophen ingestion, activated charcoal can be used to prevent the absorption of acetaminophen in the gut.
In ALF due to acetaminophen toxicity, liver transplantation is firmly indicated in patients with grade III or IV hepatic encephalopathy, PT >100 seconds, and serum creatinine >3.4 mg/dL (as in this patient). One-year survival following liver transplantation for ALF is approximately 80%.
Fulminant Liver Failure
Liver failure occurs when the liver is unable to fulfill its synthetic and metabolic functions, resulting in systemic symptoms or failure of other organ systems.
Acute liver failure occurs within weeks or monthsof severe acute liver injury, such as acetaminophen poisoning.
Chronic liver failure or cirrhosis is due to repeated liver injury, most commonly due to hepatitis C.
Fulminant hepatic failure is the rapid onset of acute liver failure and hepatic encephalopathy within 2 months of symptom onset.
Management is aimed at supportive care, stabilizing the patient, and treating the complicationsof their liver injury.
Causes of fulminant hepatic failure include:
Toxins(e.g. acetaminophen toxicity)
Viruses (e.g. Hepatitis B)
Ischemia
Metabolic disorders
Symptoms of liver failure can be vague or multi-systemic, and include:
Weight loss
Anorexia
Weakness
Jaundice and scleral icterus
Palmar erythema
Spider nevi
Gynecomastia
Hepatic encephalopathy (manifested as asterixis, confusion)
Coagulopathy
Anemia
Lower extremity edema
Specific symptoms are generally the result of either metabolic compromise or synthetic compromise of the liver. Metabolic compromise can lead to:
Hyperbilirubinemia
Jaundice
Hyperestrogen state (palmar erythema, spider angioma, gynecomastia, testicular atrophy)
Synthetic compromise may lead to:
Hypoalbuminemia and edema
Coagulopathy
Management of liver failure focuses on the preservation of remaining function and avoidance of further injury.
Medications that can be used for the complications of liver failure include:
Lactulose and rifaximin for hepatic encephalopathy
Cefotaxime for spontaneous bacterial peritonitis
Bile acid sequestrants(i.e. colestipol and cholestyramine, etc) for jaundice causing pruritis
Salt restriction and diuretics to prevent volume overload from ascites
Additional ways liver failure can be managed include:
Avoidance of hepatotoxins, especially drugs cleared by the liver
High protein diet and B vitamins to support metabolic function
The most serious complication of liver failure is death. This is typically due to one of the following:
Progressive liver failure
Complications of portal hypertension such as rupture of esophageal varices or hepatic encephalopathy
Development of hepatocellular carcinoma
Transplantis the only curative treatment for liver failure.
Cirrhosis
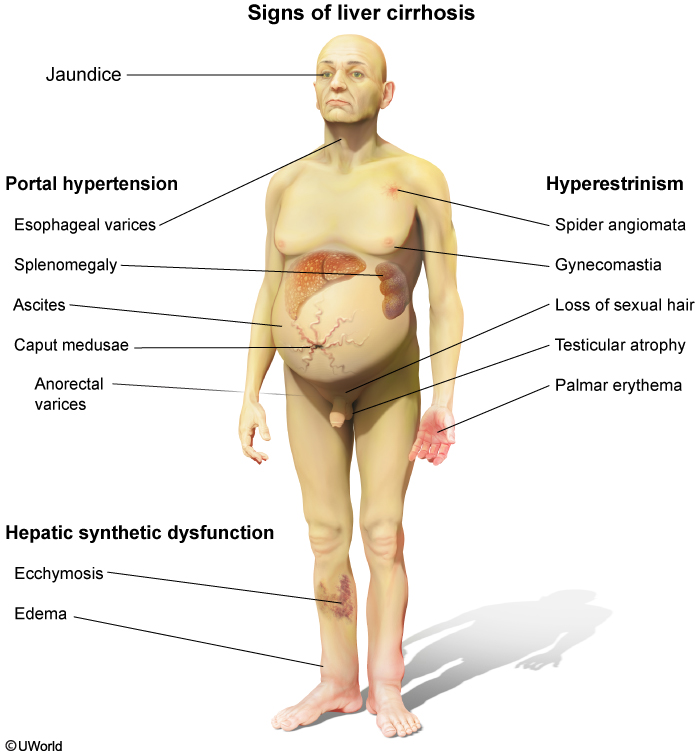
Cirrhosis is a diffuse, irreversible hepatic process characterized by fibrosis, regenerating nodules, and distortion of the normal hepatic parenchyma.
Some common causes of cirrhosis within the U.S. are:
Hepatitis C (most common)
Alcoholic liver disease (2nd most common)
Non-alcoholic fatty liver disease (NAFLD)
Hepatitis B
An acute/chronic disease process injures the liver cells, which then undergo inflammatory changes leading to necrosis and fibrosis. Sub-endothelial fibrosis leads to the impairment of normal hepatic functionality.
Less common causes of cirrhosis include:
Autoimmune hepatitis
Primary biliary cirrhosis
Primary sclerosing cholangitis
Hemochromatosis
Wilson’s disease
Alpha-1 antitrypsin deficiency
Drug induced causes
Patients with cirrhosis present with several physical findings including:
Jaundice
Palmar erythema
Spider angiomata
Petechiae
Ascites
Hepatic encephalopathy
Gynecomastia
Caput medusa
Asterixis
Physical exam of the liver may reveal an enlarged, normal, or small sized liver. If palpable, cirrhosis causes it to have a firm and nodular consistency.
Compensated cirrhosis patients will classically present with nonspecific symptoms such as:
Fatigue
Weight loss
Weakness
Decompensated cirrhosis patients may present with more specific symptoms, including:
Confusion
Ascites
Edema
Pruritus
Hematemesis
Melena
Diagnosis
The gold standard for diagnosing cirrhosis is a liver biopsy, however it is not necessary if clinical, laboratory and radiographic studies suggest cirrhosis. The pathologic features of cirrhosis include: the presence of fibrosis, regenerating hepatic nodules, and decreased number of septa.
Ultrasound is the most common imaging in the evaluation of cirrhosis, as it allows the physician to assess the contour of the liver, impedance of blood flow, and amount of ascites within the abdomen. Other helpful imaging modalities include CT abdomen, radiographs (chest and kidney-ureter-bladder views to assess for secondary complications of cirrhosis), and MRI.
In the work up of cirrhosis, there are several laboratory tests that are required to assess the functionality of the liver. Common laboratory tests include:
Bilirubin
Aspartate aminotransferase (AST)
Alanine aminotransferase (ALT)
Gamma-glutamyl transpeptidase (GGT)
Albumin
Prothrombin time (prolonged in cirrhosis)
Platelet count (low in cirrhosis)
Electrolyte panel (low sodium)
Patients with cirrhosis are susceptible to several complications including:
Ascites
Spontaneous bacterial peritonitis
Variceal hemorrhage
Hepatopulmonary syndrome
Hepatorenal syndrome
Portal vein thrombosis
Cardiomyopathy
Hepatic encephalopathy
Cirrhosis (especially due to alcoholic liver disease or hemochromatosis) can cause hypogonadism due to primary gonadal injury or hypothalamic-pituitary dysfunction. Cirrhosis is also associated with elevated circulating levels of estradiol due to increased conversion from androgens. Findings due to excess estrogen include telangiectasias, palmar erythema, testicular atrophy, and gynecomastia (usually bilateral but can be unilateral).
In addition, the liver produces serum binding proteins for thyroid hormones (eg, thyroxine-binding globulin, transthyretin, albumin, lipoproteins). Cirrhosis leads to decreased synthesis of these proteins, which lowers the total triiodothyronine (T3) and thyroxine (T4) in circulation; however, free T3 and T4 levels are unchanged, and TSH will be normal, reflecting a euthyroid status.
Portal hypertension (PHT) is the underlying cause of most of the complications associated with cirrhosis. As the pressure becomes severely elevated venous blood begins to back up into the esophageal and gastric veins, resulting in varices and even ascites.
Treatment
The primary focus in treating cirrhosis is treatment of the underlying disease process. This involves managing toxic insults and preventing serious secondary complications.
Lactulose is used during acute hepatic encephalopathy. Lactulose prevents the absorption of ammonia. It is theorized that ammonia and other neurotoxic metabolites build up in the bloodstream and gain entry into the brain secondary to liver failure.
Transjugular intrahepatic portosystemic shunt (TIPS) is a procedure performed when patients present with severe portal hypertension. This procedure creates a new route for blood to pass through the damaged liver.
Beta blockers are used to help keep the pressure low when a patient presents with esophageal or gastric varices.
Ultimately, transplantation is the only cure for a cirrhotic liver. Before transplantation is considered, the patient’s prognosis is calculated using either the Child-Pugh classification or the Model for End Stage Liver Disease (MELD).
Hemochromatosis
Hemochromatosis is the abnormal accumulation of iron in parenchymal organs, leading to organ toxicity.
Primary (hereditary) hemochromatosis is a result of autosomal recessive inheritance in a mutation of the HFE gene on chromosome 6. This leads to excessive iron absorption and progressive iron overload.
The secondary form of hemochromatosis may occur in the following situations:
Conditions resulting in ineffective erythropoiesis (e.g. thalassemia, sideroblastic anemia)
Multiple transfusions
Chronic liver disease
Hemochromatosis is associated with the HLA-A3 haplotype.
Onset is in adult years (30-50 yrs) with the classic triad of:
Micronodular cirrhosis (deposition of hemosiderin in the liver)
Diabetes mellitus(iron accumulation in pancreas and beta cell damage)
Bronze skin pigmentation(secondary to hemosiderin and melanin deposition)
Other symptoms may include:
Arthropathy(40-60% of patients, may be the first sign of disease)
Chondrocalcinosis
2nd and 3rd metacarpophalangeal (MCP) arthritis
Dilated or restrictive cardiomyopathy (iron accumulation in the myocardium)
Amenorrhea, impotence, hypogonadism
The best screening test is transferrin saturation, while serum ferritin is used to follow therapy. Both values are elevated.
Iron studies will reveal:
Increased serum iron
Increased serum ferritin
D ecrease in total iron binding capacity
Due to the abundance of iron, transferrin % saturation is increased
If performed, liver biopsy reveals high iron content seen with Prussian blue staining.
Genetic testing for HFE gene mutations is pivotal for diagnosis.
Complications include:
Hepatocellular carcinoma
Cirrhosis
Congestive heart failure
Cardiac arrhythmias
Hypogonadism
Arthropathy
Diabetes mellitus
Thyroid dysfunction
Sepsis
Medical treatment for hereditary hemochromatosis includes repeated phlebotomy (preferred) or iron chelating agents (e.g. deferoxamine, deferiprone, deferasirox).
Surgery is indicated for end-stage liver disease (transplant) and severe arthropathy (arthroplasty).
Wilson Disease
Wilson disease is an autosomal recessive disordercharacterized by impaired biliary copper secretion, leading to copper deposition in the liver, brain, eyes, and other tissues.
Wilson disease usually presents in patients < 30 years of age.
In contrast, hereditary hemochromatosis usually presents after age 40 in males and even later in females.
ATP7B encodes a transmembrane copper transporter. Mutation of this gene in Wilson disease causes:
Decreased copper secretion into bile
Decreased copper incorporation into ceruloplasmin
Decreased ceruloplasmin secretion into blood
The defect involves a copper transporting protein (ATP7B) and is linked to chromosome 13.
Physical exam findings in a patient with Wilson disease may include:
Kayser-Fleischer rings in the cornea (pathognomonic)
Hepatomegaly
Parkinsonian tremor
Slit-lamp examination of patients with Wilson disease classically reveals Kayser-Fleischer rings, which are green/brown deposits of copper in the Descemet membrane of the cornea.
Patients with Wilson disease often present with movement disorders that resemble Parkinson disease due to copper deposition in the basal ganglia, in particular the putamen. Copper deposition in other parts of the CNS can cause dementia, dyskinesia, and dysarthria.
Liver symptoms may include:
Asterixis
Jaundice
Mental status changes (secondary to hepatic encephalopathy)
Acute hepatitis
Portal hypertension
Coombs-negative hemolytic anemia can occur in Wilson disease since copper is toxic to red blood cell membranes.
Copper deposition in the joints causes degenerative arthritis, typically affecting the spine and large joints.
Wilson disease is diagnosed in the following ways:
Neurologic symptoms + Kayser-Fleischer rings + ceruloplasmin <20 mg/dL
OR
Isolated liver disease + hepatic copper concentration >250 mcg/g dry weight liver + low serum ceruloplasmin
If neurologic symptoms and Keyser-Fleischer rings are absent, liver biopsy and quantitative copper determination is necessary for diagnosis.
Genetic testing is not required for diagnosis. Genetic studies are useful for screening family members of affected patients in whom there has been a previously identified mutation.
Patients with Wilson's disease will have decreased serum ceruloplasmin with elevated free copper but decreased total copper.
The mainstay of treatment for Wilson disease is pharmacologic treatment with chelating agents, including zinc and D-penicillamine.
Medications used to treat the other symptoms of Wilson disease include:
Anticholinergics, GABA antagonists, and levodopa(for treatment of parkinsonism)
Antiepileptics (to treat seizures)
Neuroleptics (to treat psychosis and other psychiatric symptoms)
Lactulose (to treat hepatic encephalopathy)
Orthotopic liver transplantation is curative for Wilson disease and may be indicated in the case of worsening hepatic function or progression of disease.
Complications of Wilson disease include:
Cirrhosis
Liver failure
Persistent neurological deficits (Parkinsonian symptoms, dementia)
Psychiatric problems (personality changes, depression, bipolar disorder, psychosis)
Amino aciduria
Nephrocalcinosis
Hepatic Encephalopathy
Hepatic encephalopathy occurs when ammonia clearance is compromised (due to chronic liver dysfunction) and consequently builds up and crosses into the CNS.
Patients present with altered mental status(e.g. confusion, stupor, coma) and signs of chronic liver disease (e.g. asterixis).
Diagnosis is based on serum ammonia levels, and evaluating liver function via the following:
AST
ALT
Albumin
Bilirubin
Prothrombin time
Partial thromboplastin time
Treatment is aimed at ammonia reduction, which can be achieved through:
Lactulose,which prevents the absorption of ammonia
Antibiotics (i.e. rifaximin or neomycin) that decrease ammonia production by gut bacteria
Diet adequate with protein and energy (low protein diet, though once considered beneficial, has been shown to be detrimental in most patients as they are already malnourished and require sufficient protein to maintain a healthy body weight)
Branched-chain amino acid and probiotic supplementation, if indicated
Patients with advanced liver disease should be evaluated for transplant as this is the only curative measure.
Hepatorenal Syndrome
Hepatorenal syndrome is the development of renal failure in patients with acute or chronic liver disease. Hepatorenal syndrome most often occurs in patients with decompensated cirrhosis and severe alcoholic hepatitis, but can occur in anyone with severe liver disease.
In the setting of severe cirrhosis of the liver, portal hypertension induces the aberrant production of the vasodilator nitric oxide (NO) within the splanchnic circulation. A NO-mediated reduction in vascular resistance within the splanchnic circulation results in a fall in systemic vascular resistance, which causes renal hypoperfusion and triggers the activation of the renin-angiotensin-aldosterone and sympathetic nervous systems.
These compensatory neurohormonal responses further exacerbate renal hypoperfusion and fluid retention.
Patients with hepatorenal syndrome present with clinical symptoms of kidney failure such as:
Azotemia
Oliguria
Hyponatremia
Hypotension
Diagnosing hepatorenal syndrome is one of exclusion. Other causes of acute kidney injury such as glomerulonephritis, ATN and prerenal disease must be ruled out before a diagnosis of hepatorenal syndrome can be made.
Patients with hepatorenal syndrome have laboratory findings consistent with prerenal azotemia:
Low urine sodium (<10 mEq/L)
FeNa <1%
BUN:Cr ratio >20:1
Serum Cr >1.5
Hepatorenal syndrome may be distinguished from prerenal azotemia through a fluid challenge.
In prerenal azotemia, the patient's condition will improve upon administration of a fluid bolus.
In hepatorenal syndrome, fluid bolus administration will exacerbate the condition through initially expanding the intravascular space, and subsequently expanding the "third space".
Hepatorenal syndrome is considered a functional renal failure since the kidneys are morphologically and histologically NORMAL. Therefore, the definitive treatment for patients with hepatorenal syndrome is liver transplant rather than kidney transplant.
Treatment
Hepatorenal syndrome is best managed with vasoconstrictors such as:
Terlipressin
Norepinephrine
Octreotide
Midodrine
Note: Midodrine is a systemic vasoconstrictor, and octreotide inhibits vasodilator release. When given in combination with albumin, this therapy improves renal hemodynamics.
Patients with hepatopulmonary syndrome have hypoxemia and dyspnea in the presence of long standing liver disease.
Two exam findings specific to patients with hepatopulmonary syndrome are:
Orthodeoxia is hypoxia in an upright or sitting position that improves when returning to a recumbent position.
Platypnea is increased dyspnea while walking in an upright position that is relieved when returning to a recumbent position.
Alcoholic Liver Disease
Alcoholic liver disease is a disorder that causes progressive liver inflammation and damage from excessive alcohol intake.
The progression of liver disease in alcoholics if untreated goes from fatty steatosis, to alcoholic hepatitis to eventually liver cirrhosis/fibrosis. If the patient receives treatment, fatty steatosis and alcoholic hepatitis are usually reversible. Changes associated with cirrhosis and liver fibrosis, however, are not.
Most patients with alcoholic liver disease will experience non-specific symptoms, such as fatigue, malaise, and nausea. Patients who have cirrhosis due to alcohol liver disease can develop any of the following conditions:
Jaundice
Ascites
Esophageal varices
Spider angiomata
Gynecomastia
Testicular atrophy
Dupuytren contracture
Diagnosis of alcoholic liver disease is made after excluding other causes of cirrhosis or fatty liver, and includes:
Detailed history about alcohol use, including amount and frequency
Physical examto look for stigmata of chronic liver disease
Blood work to assess liver synthetic function and inflammation (including CBC, albumin, AST/ALT, bilirubin, alkaline phosphatase, coagulation studies)
Ultrasound of the liver
A liver biopsy may be required if diagnosis can't be reached via noninvasive methods. Biopsy may reveal eosinophilic hyaline inclusion bodies (Mallory bodies), steatosis (fatty deposition), and fibrosis.
A ratio of aspartate aminotransferase (AST) to alanine aminotransferase (ALT) greater or equal to 2:1 is highly associated with alcoholic liver disease. Other causes of liver disease rarely produce similar elevations in liver enzymes.
Other laboratory tests that are commonly elevated in alcoholic liver disease include:
Bilirubin
Alkaline phosphatase
Gamma-glutamyl transpeptidase (GGT)
PT/INR
MCV
Alcoholic liver disease may result in:
Cirrhotic complications (eg jaundice, hematemesis, portal hypertension, encephalopathy)
Liver failure
Hepatocellular carcinoma
Death
Abstinence is the main focus in treating patients without end stage liver disease. Supportive care in the way of proper nutrition and immunization (Hep A, Hep B) prevent further hepatic damage.
Medications such as Disulfiram (Antabuse) can assist patients in the abstinence process. Disulfiram works by inhibiting the action of acetaldehyde dehydrogenase responsible for the conversion of acetaldehyde to acetic acid in the second step of the metabolism of ethanol. Increased levels of acetaldehyde causes flushing, nausea and vomiting when alcohol is consumed.
Drug Induced Hepatitis
The liver is especially vulnerable to drug toxicity as it metabolizes nearly all medications and herbal supplements via the cytochrome P-450 system. The clinical manifestations of drug induced liver injury are highly variable. Patients may present with abnormal LFTs and be otherwise asymptomatic or in severe cases, patients may present with fever, malaise, anorexia, RUQ pain and jaundice.
Drugs and toxins typically cause hepatic injury, either through direct toxic effects or through idiosyncratic reactions. The direct toxic effects are dose-dependent and have short latent periods. Some examples of direct toxins include carbon tetrachloride, acetaminophen, tetracycline, and substances found in the Amanita phalloides mushroom. Idiosyncratic reactions are not dose-dependent and have variable latent periods. Some examples of pharmacological agents that cause idiosyncratic reactions include isoniazid, chlorpromazine, halothane, and antiretroviral therapy.
Drug-induced liver disease can also be broadly categorized according to morphology: 1) cholestasis, which is caused by medications such as chlorpromazine, nitrofurantoin, erythromycin, and anabolic steroids; 2) fatty liver, which is caused by medications such as tetracycline, valproate, and anti-retrovirals; 3) hepatitis, which is caused by medications such as halothane, phenytoin, isoniazid, and alpha-methyldopa; 4) toxic or fulminant liver failure, which is caused by medications such as carbon tetrachloride and acetaminophen; and 5) granulomatous, which is caused by medications such as allopurinol and phenylbutazone.
Oral contraceptives are unusual in that they can cause abnormalities in liver function tests without evidence of necrosis or fatty change. Since this patient's liver biopsy demonstrated necrosis, oral contraceptives are not likely responsible for her hepatic dysfunction.
Acetaminophen is the most common cause of drug induced liver injury in the U.S.
Other hepatotoxic medications include:
Statins
Antibiotics - amoxicillin-clavulanate, isoniazid
Amiodarone
Antifungals
Valproic acid
The diagnosis of DILI is one of exclusion. Physicians must take a careful drug use history to rule out other potential causes of hepatotoxicity.
Patients with suspected drug induced liver toxicity should be worked up in a similar fashion to those with other forms of liver disease. Performing hepatic function tests such as AST, ALT, bilirubin, alkaline phosphatase, and albumin are essential in the work up. Patients should also receive a hepatitis panel.
Treatment for drug induced liver injury is primarily supportive and includes early recognition of liver damage, withdrawal of the offending agent, and appropriate use of an antidote if available.
PVT
Portal vein thrombosis (PVT) occurs when the portal vein (formed by the fusion of the splenic and superior mesenteric veins) becomes obstructed by a thrombus. When the portal venous system becomes obstructed, blood tends to back up into the surrounding veins, resulting in complications such as portal hypertension.
Inherited disorders that predispose patients to PVT include:
Factor V leiden mutation
Protein C and S deficiency
Antithrombin deficiency
Acquired disorders which can cause PVT include:
Cirrhosis
Sepsis
Hepatobiliary malignancy
In portal vein thrombosis, patients may present with right upper quadrant pain, nausea, and vomiting. Patients may also suffer from complications associated with portal hypertension including variceal bleeding and splenomegaly. Ascites is found infrequently, but may occur with underlying liver disease. Symptoms may differ in severity based on the extent and duration of portal vein occlusion.
Complications from liver cirrhosis can present similarly to patients presenting with PVT since both conditions cause portal hypertension. If a patient has normal LFTs, splenomegaly and varicosities WITHOUT ascites and changes in the hepatic parenchyma, PVT should be high on the differential diagnosis.
The best initial screening test for a patient with suspected portal vein thrombosis is Doppler ultrasound.
Contrast-enhanced magnetic resonance angiography (MRA) or contrast enhanced CT is used to definitively diagnose PVT.
Portal vein thrombosis may result in:
Intestinal ischemia
Septic portal vein thrombosis
Gastroesophageal varices
In patients diagnosed with PVT, treatment with anticoagulation therapy with low-molecular weight heparin (LMWH)is warranted. Once stabilized, the patient is switched to warfarin.
Hepatitis
Overview
Viral hepatitis is an infection causing inflammation of the liver, hepatocyte injury and necrosis.
There are four main RNA viruses that cause hepatitis:
Hepatitis A virus (HAV)
Hepatitis C virus (HCV)
Hepatitis D virus (HDV)
Hepatitis E virus (HEV)
HAV is an RNA picornavirus that is transmitted via the oral-fecal route, and leads to acute hepatitis. It has no chronic carrier state.
HCV is a flavivirus that is transmitted via blood and leads to acute hepatitis. The chronic carrier state (60-80% of patients) can lead to hepatocellular carcinoma.
HDV is a delta virus that is transmitted by blood, and needs HBV for co-infection or superinfection.
Mnemonic: HDV is a Defective RNA virus that requires HBV for replication.
HEV is transmitted via the fecal-oral (enteric) or waterborne routes and leads to acute hepatitis. It is more virulent during pregnancy. Remember HEV, Efor Enteric.
Hepatitis A and E are acquired via oral-fecal transmission (HAV, HEV). A useful mnemonic is: “Vowels Hit the Bowels”.
There is one DNA virus that causes hepatitis: Hepatitis B virus (HBV). It is a Hepadnavirus that is transmitted by blood. Only ~10% become chronic carriers (↑ chance if perinatal infection), which can lead to cirrhosis and hepatocellular carcinoma.
Acute hepatitis may present with:
Nausea
Vomiting
Malaise
Fever
Jaundice
Abdominal pain
Acute viral hepatitis has a variable incubation period. It presents with increased AST and ALT and moderately elevated GGT and alkaline phosphatase. Compare this to jaundice due to obstruction, which would have markedly elevated GGT and alkaline phosphatase and only moderately increased AST and ALT.
Chronic hepatitis may present with jaundice and hepatomegaly.
Chronic viral hepatitis is defined as:
Active disease that persists for more than 6 months without recovery OR
Asymptomatic carrier which “carries” the virus and doesn’t develop injury (due to lack of anti-surface antibody production)
Asymptomatic carriers may have slightly elevated AST and ALT and possible hepatomegaly.
Hepatitis B, C and D are transmitted via blood, can develop into chronic carriers, and can lead to cirrhosis and/or hepatocellular carcinoma.
The diagnosis of hepatitis is based on serology.
The presence of antibodies against Hepatitis A indicates either an acute infection or immunity. The presence of IgM antibody, however, is specific for an acute infection.
Hepatitis B serology must be interpreted to diagnose the state of infection:
The presence of Hepatitis B surface antigen indicates either an acute or chronic infection.
The presence of Hepatitis B e antigen indicates an active and infectious virus.
The presence of anti-Hepatitis B surface antigen indicates either a prior infection or immunization.
The presence of anti-Hepatitis B core antigen IgM indicates an acute infection.
The presence of anti-Hepatitis B core antigen IgG indicates a chronic infection.
The Hepatitis B viral load can be checked by PCR as an indicator of the extent of viral activity.
HBsAg
HBeAg
Anti-HBc IgM
Anti-HBc IgG
Anti-HBs
Susceptible
-
-
-
-
-
Previous Infxn
-
-
+
+
+
Immunized
-
-
-
-
+
Acute Infxn
+
+
+
-
-
Chronic Infxn
+
+
-
+
-
The presence of antibodies against Hepatitis C and positive HCV polymerase chain reaction indicates either an acute or chronic infection.
The presence of antibodies against Hepatitis D indicates either an acute or chronic infection.
Treatment and prophylactic measures for viral hepatitis varies depending on type and severity, and may include:
Rest (as many cases are self-limited)
Interferon alpha or antivirals for Hepatitis B infection
Interferon alpha with or without ribavirin for Hepatitis C infection
Immunoglobulin given to close contacts of patients with Hepatitis A, or unvaccinated patients that have been exposed to Hepatitis B
Hepatitis A vaccine given to patients traveling to endemic areas
Hepatitis B vaccine for healthcare workers and children
Patients with chronic liver disease or impending liver failure due to hepatitis should be considered for liver transplant.
Complications of viral hepatitis include:
Chronic hepatitis
Cirrhosis
Hepatocellular carcinoma
Persistent carrier state
Fulminant hepatic failure
Death
These each depend on the type and severity of the infection.
Hep A
Hepatitis A (HAV) is one of the more common causes of acute hepatitis. Hepatitis A is caused by a non-enveloped, single-stranded picornavirus. The primary route of infection is almost always fecal-oral, which typically results in bilirubinuria, jaundice, and abdominal pain.
The U.S. has a much lower incidence of the virus compared to Mexico and Central/South America. Other risk factors for contracting the virus (in addition to foreign travel) include:
Personal contact
Consumption of shellfish
Day-care centers
Institutionalization (e.g. prisoners)
Male homosexuality
Patients initially present with a prodromal stage (i.e. fever and flu-like symptoms), often progressing to the icteric stage (i.e. bilirubinuria, pale stools, and jaundice).
The diagnostic modality of choice for hepatitis A is serologic testing, which tests for IgM antibodies to the hepatitis A virus. Anti-HAV IgG without the presence of IgM indicates prior vaccination or past infection.
Hepatosplenomegaly, abdominal pain, and jaundice are extremely common on physical exam.
Diagnosis
Liver function tests such as AST and ALT are good indicators of an HAV infection, which can exceed 10,000 mIU/mL. Bilirubin levels will also become elevated.
HAV may result in complications such as:
Prolonged or relapsing symptoms
Acute liver failure
Death (more likely in patients with concomitant Hepatitis C infection, or in the elderly)
Patients do not develop chronic liver disease as a result of hepatitis A.
The disease is usually self-limited and the treatment is generally supportive (i.e. hydration), as most patients (85%) recover completely within 6 months.
Vaccination is available for patients prior to traveling to endemic areas.
Hep D
Hepatitis D virus (HDV) infection is an acute and chronic inflammatory process of the liver, which requires the presence of the hepatitis B virus (HBV) to propagate, thus HDV infection always occurs in association with HBV infection.
HDV infection is transmitted percutaneously, sexually and (rarely) perinatally.
HDV infection most commonly occurs in intravenous drug users and is endemic in the Mediterranean basin.
Other risk factors include blood transfusions, high-risk sexual activity, and perinatal transmission.
Many patients are asymptomatic, but symptoms to watch for include:
Abdominal pain
Jaundice
Ascites
Nausea
Vomiting
Dark urine
The following serologic studies help confirm a hepatitis D infection:
Reverse transcriptase PCR for HDV RNA (most sensitive)
anti-HDV IgM (initially) and anti-HDV IgG
anti-HBc IgM
HBsAg (may be suppressed or undetectable during active HDV replication)
Additional laboratory findings may include elevated AST and ALT (above 500 IU/L), INR greater than 1.5, or elevated prothrombin time.
Complications of HDV may include:
Severe hepatitis
Chronic carrier state
Cirrhosis
Encephalopathy
Fulminant liver failure
One of the most feared complications of HDV is a superinfection, which occurs when a patient becomes infected with the hepatits D virus who is already positive for the hepatitis B surface antigen(ie chronic carrier or acute HBV infection). These patients can progress to fulminant liver failure very rapidly.
Treatment is primarily supportive in HDV infection. The patient's liver enzymes and mental status should be observed very carefully. In the case of chronic infection, treatment with interferon alfa may be considered.
Increasing liver enzymes and decreased mental status may be signs of fulminant liver failure, which requires liver transplantation.
Administration of the Hepatitis B vaccine is protective against Hepatitis D.
Hep E
Hepatitis E (HEV) is a single-stranded RNA virus that is transmitted via contaminated water and the fecal-oral route. The resulting liver infection is usually self-limited.
HEV is endemic in Africa, Asia, and the Middle East.
It most commonly occurs in fecally contaminated water sources within endemic areas and with ingestion of undercooked pork.
Patients typically present with 2 phases of symptoms, the prodromal phase and the icteric phase.
Prodromal symptoms include:
Myalgias
Mild fever
Right upper quadrant pain
Nausea/vomiting
Weight loss
Anorexia
Icteric phase symptoms include:
Light colored stools
Jaundice
Pruritis
The best tests in diagnosing HEV infection are serum HEV RNA and anti-HEV immunoglobulin (IgM).
Other helpful laboratory tests include:
ALT
AST
Bilirubin
Alkaline phosphatase
Elevated (10-20 times normal) aminotransferases are the hallmark in acute viral hepatitis.
HEV may result in complications such as encephalopathy, disseminated intravascular coagulation (DIC), and fulminant hepatic failure.
For unknown reasons, pregnant woman in their third trimester have an increased risk of developing fulminant hepatic failure following Hepatitis E infection.
Treatment of HEV infection is primarily supportive. Liver transplant is required in patients with fulminant hepatic failure.
Preventive measures include careful cooking of food and drinking clean water.
Hep B
Hepatitis B is a double-stranded DNA virus, which causes acute and chronic inflammation to the liver.
The virus is most commonly transmitted via bodily fluids such as blood, semen and vaginal secretions.
HBV infection most commonly occurs in:
Accidental needle sticks
Sharing needles
Sexual contact
Perinatal transmission
Common symptoms during the acute phase include:
Scleral icterus
Abdominal pain
Pruritus
Myalgias
Possible progression to fulminant hepatic failure
Patients with chronic disease may be asymptomatic or complain of:
Myalgias
Nausea
Abdominal discomfort
Mild jaundice
Diagnosis of hepatitis B is based on serological studies. The following serum markers are positive during acute hepatitis B infection:
Hepatitis B surface antigen (HBsAg)
Anti-HBV core antibody IgM (anti-HBc)
HBeAg (marker of infectivity)
As long as HBeAg is in the serum, patients can still infect others.
The following table summarizes the descriptions of the hepatitis B serology markers:
HBsAg
Surface antigen, indicates active infection or carrier state (HBsAg: virus is preSent)
HBsAb
Antibody against HBsAg, presence indicates immunity to HBV
HBcAg
Core antigen
HBcAb
Antibody against core antigen. Critical for diagnosis during the window phase, when HBsAg is absent but HBsAb isn't detectable yet. Doesn't confer immunity.
HBeAg
Core antigen. Presence indicates transmissibility (HBeAg=rEplication)
HBeAb
Antibody against HBeAg, indicates low transmissibility
In general, chronic hepatitis B infection is characterized by positive HBsAg and anti-HBc IgG antibody. Presence of HBsAg for longer than 6 months alone is indicative of chronic infection.
Inactive carriers of the HBV are negative for HBeAg, but positive for HBsAg.
Prior HBV infection is characterized by anti-HBs (IgG) and anti-HBc (IgG).
Patients immunized will only be positive for anti-HBs antibodies.
Liver biopsy is not always required, but helps determine the extent of damage to the liver.
Hepatitis B viral infection may result in:
Cirrhosis
Hepatocellular carcinoma
Renal failure
Spontaneous bacterial peritonitis
Fulminant hepatic failure
In unvaccinated patients, immediate treatment involves administration of the Hepatitis B vaccine and Hepatitis B immune globulin following exposure to HBV.
Pharmacologic therapy is recommended for patients with chronic active hepatitis B (positive HBV DNA, elevated liver enzymes, and positive HBeAg), acute hepatic failure, and cirrhotic patients. First-line agents include: pegylated interferon alpha (PEG-IFN-a),entecavir, andtenofovir. Other agents such as lamivudine (3TC), telbivudine, and adefovir are second- or third-line agents.
Routine vaccination is routinely given to healthcare workers and children.
Hep C
Hepatitis C virus (HCV) is a single-stranded RNA flavivirus.
HCV is transmitted via blood, and possibly sexual contact. Risk factors include:
Intravenous drug users
Accidental needle stick
Sexual intercourse with an infected individual
Blood transfusions
Perinatal transmission
Infection can result in both acute and chronic inflammation of the liver. The acute process is typically self-limited, while a chronic HCV infection is more progressive in nature, which can lead to cirrhosis, hepatocellular carcinoma, and even liver failure.
The best initial diagnostic test is a HCV antibody test (anti-HCV), which looks for antibodies against the virus within the patient’s serum.
The best test for confirmation of infection is qualitative PCR, which detects whether the virus is in the patient’s blood.
Other tests include quantitative HCV testing (detects amount of virus within blood) and genotype testing. This helps determine both the type and length of treatment, as some genotypes are more susceptible to certain medications.
Most patients with chronic HCV infection are asymptomatic at the time of diagnosis. Symptomatic patients may present with:
Fatigue/malaise
Myalgias
Jaundice
Nausea
Vomiting
Weight loss
Other conditions that are associated with Hepatitis C infection include:
Mixed cryoglobulinemia
Lymphoma
Thyroiditis
Polyarteritis nodosa(more commonly associated with Hepatitis B)
Membranous nephropathy
Membranoproliferative glomerulonephritis
Porphyria cutanea tarda
Lichen planus
In patients with HCV, mixed cryoglobulinemia is diagnosed by:
Palpable purpuraor other skin manifestations
Biopsy can confirm diagnosis
Low complement levels
Circulating serum cryoglobulins
Patients with mixed cryoglobulinemia may also have elevated rheumatoid factor titers.
Patients with either mixed cryoglobulinemia or porphyria cutanea tarda should be screened for HCV.
Complications of HCV infection may include:
Chronic hepatitis (80% of patients)
Cirrhosis(in 50% of those with chronic disease)
Hepatocellular carcinoma
Persistent carrier state
Liver failure
Death
The current standard of care for treatment of patients with HCV is pegylated interferon and ribavirin.
The newest 2014 recommendations from the American Association for the Study of Liver Diseases and the Infectious Disease Society of America include the addition of a polymerase inhibitor(sofosbuvir) to the PEG-IFN/RBV combination in the treatment of chronic HCV.
Sofosbuvir is also now available in combination with ledipasvir(combination trade name Harvoni) for treatment of disease caused by genotype 1. It does not require coadministration with interferon or ribavirin.
Once diagnosed, patients should wait at least 12 weeks before starting therapy to allow spontaneous clearance.
Quantitative HCV tests (i.e. viral load) are carried out to assess response to treatment. The timing of these are dependent on the serotype of virus, and may involve testing after 4 weeks of treatment, 12 weeks of treatment, and/or at the cessation of treatment.
If patients do not eradicate the virus, it is recommended not to retreat them. These patients will be monitored for progressive fibrosis and evaluated for liver transplantation.
All patients with chronic hepatitis C should receive vaccination against Hepatitis A and B if not already immune.
Colon
IBS
Irritable bowel syndrome (IBS) is a benign idiopathic disorder characterized by abnormal activity of the GI tract while at rest.
Other associated findings include depression and anxiety.
Physiologic symptoms include a combination of:
Diarrhea
Constipation
Abdominal pain
Abdominal bloating
IBS symptoms are notably relieved by defecation.
IBS may be classified as:
C onstipation-predominant
D iarrhea-predominant
A lternating
P ost-infectious
Certain red-flag symptoms should prompt workup for a more serious condition. These include:
Anemia
Bleeding
Weight loss
Early satiety
The Rome IV criteria for irritable bowel syndrome are used for diagnosis. They specify that the patient must have:
Recurrent abdominal pain for at least 1 day per week in the last 3 months PLUS two or more of the following:
Related to defecation
Associated with a change in stool frequency
Associated with a change in stool form (appearance)
Irritable bowel syndrome is a diagnosis of exclusion. Other etiologies should be ruled out first with lab studies and stool exams, and clinical suspicion for IBS is needed.
Differential diagnosis includes:
Inflammatory bowel disease
Celiac disease
Depression
Somatization
GI malignancy
Obstruction
Malabsorption
Infectious etiologies
Treatment is usually based upon dietary and lifestyle modifications for symptoms relief. These may include:
Lactose free diet
Exclusion of gas producing foods
Judicious water intake (for predominant constipation)
Fiber supplementation
Avoidance of caffeine to limit anxiety and symptom exacerbation
Anti-spasmodic or anti-cholinergic drugs may be used as adjunctive treatment. The best treatment remains stress management.
When medications are indicated, treatment options include:
Diarrhea-predominant
Antidiarrheals (e.g. loperamide, diphenoxylate hydrochloride+atropine sulfate)
Serotonin receptor antagonists (e.g. alosetron)
Antibiotics (e.g.rifaximin) for severe IBS without constipation that is refractory to other treatments
Tricyclic antidepressants (e.g. imipramine, amitriptyline)
Antispasmodic agents (e.g. dicyclomine hydrochloride, hyoscyamine sulfate)
Constipation-predominant
Laxatives(e.g. lubiprostone, linaclotide, methylcellulose)
Chloride channel activators
Guanylate cyclase C agonists
Celiac Sprue
Celiac sprue (i.e. gluten intolerance) is an immune-mediated destruction of the mucosa of the small bowel.
Pathogenesis involves the ingestion and biochemical breakdown of gluten (found in wheat, barley, rye), and the subsequent autoimmune inflammatory reaction to gliadin, an alcohol-soluble fraction of gluten. This inflammatory reaction damages the gut mucosa, causing malabsorption.
Celiac sprue has a strong hereditary component--HLA haplotypes DQ2 and DQ8 are strongly linked to disease. These HLA molecules present gliadin to helper T cells, which mediate inflammatory damage.
Other than disease in a first-degree relative, risk factors for celiac sprue include:
Presence of another auto-immune condition (e.g., type 1 diabetes mellitus, thyroid and liver disease)
Down syndrome
Characteristically, celiac disease manifests during infancy and before school age. In the classic form of childhood celiac disease, symptoms and signs of malabsorption become obvious within some months of starting a gluten-containing diet.
Children may present withfailure to thrive, and proximal muscle wastingmay be seen. In up to one quarter of children diagnosed, Rickets may be a presenting symptom.
Gastrointestinal symptoms of celiac sprue include:
Foul-smelling diarrhea
Abdominal pain
Weight loss
Bloating
Fatigue
Steatorrhea
Extraintestinal manifestations of celiac sprue include:
Anemia
Neurologic symptoms (motor weakness, paresthesias)
Dermatitis herpetiformis
Hormonal disorders (amenorrhea/infertility in women, impotence/infertility in men)
For suspected celiac disease, the best initial test is a serum level of immunoglobulin A anti-tissue transglutaminase antibody (IgA TTG). Other antibodies that can be tested include anti-endomysial and anti-gliadin. When IgA deficiency is suspected, or in children less than 2 years old, IgG anti-gliadin may also be drawn.
Other useful lab tests include:
Electrolytes (may be low, evidence of malabsorption)
Hematologic tests (anemia, low serum iron, prolonged prothrombin time)
Stool examination (fat malabsorption)
Blood cholesterol (may be low)
Vitamin D and B12 (may be low)
The gold standard in diagnosing celiac sprue is a duodenal biopsy, which shows:
Atrophic or absent villi
Hypertrophic crypts
Increased lymphocytes
Radiographic studies may be useful in untreated celiac sprue. Barium studies may show small bowel dilatation.
The primary treatment of celiac sprue is the avoidance of gluten-containing products, such as wheat, barley and rye.
Iron, folate or vitamin supplementation may be necessary until the patient is able to absorb these nutrients on his or her own.
Dermatitis herpetiformis, if present, can be treated with a gluten-free diet anddapsone.
In patients with disease refractory to treatment, corticosteroids may be helpful.
Celiac sprue may result in the following long term complications:
Anemia
Osteopenia/osteoporosis
Bleeding diathesis(increased tendency to bleed, secondary to malabsorption of fat-soluble vitamin K)
Weight loss
Severe dehydration
Malnutrition
Tropical sprue is an acquired chronic diarrheal disease similar to celiac sprue, affecting patients living in tropical areas. It may present years after the patient has left the tropical area, and is thought to be infectious or toxic in origin.
Patients present with:
Diarrhea
Steatorrhea
Weight loss
Fat-soluble vitamin deficiencies
Diagnosis relies on a combination of:
Serum findings,which may show a low-level of fat-soluble vitamins (A, D, E, K), B12, folate, and lack of anti-endomysial, anti-gliadin, and anti-tissue transglutaminase antibodies
Stool studies,showing excess fecal fat
Histologic findings on endoscopy,revealing flattening of villi and inflammation in the small bowel
It is important to rule out GI infections, autoimmune diseases, and celiac sprue prior to assigning a diagnosis of tropical sprue.
Treatment of tropical sprue consists of tetracycline plus folic acid for 3-6 months.
Unlike celiac sprue, patients with tropical sprue have no response to the removal of gluten from the diet.
Infection
Whipple
Whipple’s disease is asystemic infection caused by _Tropheryma whippelii_that typically affects the gastrointestinal tract, but may involve the heart, bones, lungs, and central nervous system.
Tropheryma whippelii (T. whippelii) is a gram-positive, periodic acid-Schiff (PAS) positive bacillus bacterium that causes Whipple's disease.
The risks associated with T. whippelii are unknown, but the bacterium is more common in sewage and wastewater.
Patients typically present with:
Weight loss
Arthralgias
Diarrhea with/without bleeding
Abdominal pain
The classical triad of symptoms is:
Arthralgia
Diarrhea
Lymphadenopathy
Oculomasticatory myorhythmia is pathognomonic for Whipple's disease. It involves eye movement disturbances along with rapidly repetitive movement of facial muscles.
Other symptoms include:
Fever
Cognitive dysfunction
Endocarditis
The best initial diagnostic test for T. whippelli infections is quantitative PCR of the stool and saliva, which helps detect bacterial load.
The diagnostic modality of choice is endoscopy with small intestine biopsy, which classically reveals PAS-positive macrophages and villous atrophy.
T. whippelii may result in complications such as:
Endocarditis
Malabsorption
Dementia
Skin hyperpigmentation
Arthritis
Steatorrhea
Nystagmus
The mainstay of treatment is antibiotic therapy for 1-year. The treatment of choice is IV ceftriaxone for 2-weeks, followed by trimethoprim-sulfamethoxazole (TMP-SMX) for 1-year.
Noncompliance with the full antibiotic course leads to high recurrence rates, and mortality if the disease is left completely untreated.
T Canis
Toxocariasis (also known as visceral larva migrans) is infection acquired from the ingestion of eggs secreted by the roundworm Toxocara canis that inhabits the small intestine of dogs.
Adult Toxacara reside within the small intestine of dogs and excrete the eggs with their stool. Once the eggs are ingested, they hatch and the larvae are able to penetrate the intestinal wallwhere they travel through the circulation and infect other portions of the body.
Most commonly occurs in young children who come into contact with dog feces at the playground or sandbox. Other risk factors include being a veterinarian and having poor hygiene.
There are 2 major syndromes associated with Toxocara canis: visceral larva migrans(VLM) and ocular larva migrans (OLM). Symptomatic presentation can vary depending on which organs the larvae come into contact with.
VLM is characterized by:
Fever
Anorexia
Hepatomegaly
Hepatitis
Pneumonitis
Urticaria
OLM is characterized by:
Unilateral decreased vision
Seeing floaters
Strabismus
Uveitis
The diagnosis of toxocariasis requires a high degree of suspicion and is dependent on serum laboratory test results. CBC may show eosinophilia. There may also be elevated IgE, and a positive ELISA antibody assay (detects antibodies against secretory antigens).
CT and ultrasound are helpful in diagnosing liver, pulmonary, and CNS lesions.
It is important to perform a fundoscopic examinationon all patients suspected of toxocariasis. Patients may present with retinal exudates, retinal detachment, and peripapillary inflammation.
Toxocara canis may result in:
Vision loss
Myocarditis
Respiratory failure
Encephalitis
Seizures
The treatment of choice in VLM and OLM is albendazole. When there is severe pulmonary, cardiac, or CNS involvement, prednisone is warranted.
Enterobius
_Enterobius vermicularis_is a pinworm and is the most common helminth in US, primarily infecting children under age 12.
_Enterobius vermicularis _is transmitted via the fecal-oral route.
The typical clinical presentation is a child under 12 with intense anal pruritis that worsens at night.
The Scotch tape test is used to recover eggs from perianal skin. It is effective due to the migration of female helminths out of the anus at night, and the deposition of eggs on the perianal skin. Eggs are NOT found in stools (vs other nematodes).
Treatment for an Enterobius vermicularis _infection is an antihelminthic for both the affected patient and all household contacts and caretakers. Mebendazole, pyrantel pamoate, and albendazole are all active against _E. vermicularis.
For successful eradication, individuals should receive three doses, three weeks apart. Reinfection is common.
Of note, all above mentioned drugs are category C for pregnant patients, and the CDC has limited data on their use during pregnancy. If medically necessary, treatment should be withheld until the 3rd trimester.
Cystoisospora
Infection with _Cystoisospora _involvesingestion of oocysts from food or water contaminated with human or animal feces of an affected host.
Diagnosis is achieved through visualization of oocysts in the feces.
Treatment of choice is trimethoprim-sulfamethoxazole. Ciprofloxacin is first line for patients with sulfa allergies.
Typical clinical presentation of a Cystoisospora infection involves an immunocompromised host with a CD4 count < 50 who has been experiencing secretory diarrhea with possible significant weight loss and wasting. It is found more commonly in tropical and subtropical regions.
Microsporidia
Microsporidia are a group of unicellular fungi (once thought to be protists)that cause microsporidiosis, a form of chronic diarrhea and wasting, in immunocompromised hosts.
Enterocytozoon species are the most well-known human pathogens among the microsporidia.
Clinical presentation involves an immunocompromised host with a CD4 < 100 presenting with watery diarrhea, fever, and abdominal pain.
Diagnosis is achieved through visualization of spores in stool samples and/or with a modified trichome stain.
Treatment is with fumagillin or albendazole.
In addition to microsporidia such as Entercytozoon species, other pathogens to consider in the patient with severe watery diarrhea include:
Gram-positive bacteria (Staph aureus, Clostridium)
Gram-negative bacteria (Vibrio cholerae, Salmonella, Campylobacter)
Viruses (rotavirus)
Parasites (cryptosporidium)
Cryptosporidium
Cryptosporidium parvum (C. parvum) is an intracellular protozoan parasite that is predominantly associated with diarrhea and biliary disease after the organism’s infectious oocysts have been ingested.
The Cryptosporidum oocysts areimmediately infectiousafter excretion. Once ingested, the oocysts undergo encystation and release sporozoites within the small bowel that attach to the epithelial cells and interfere with intestinal absorption and secretion.
Transmission most commonly occurs via the fecal-oral route from contaminated sources such as food and water.
High-risk populations include:
Immunodeficiency (e.g. AIDS)
Day-care centers
Backpackers who consume unfiltered water
People who handle infected animals
Immunocompetent infected hosts are usually asymptomatic, but may also present with:
Abdominal cramping
Watery diarrhea
Low-grade fever
Malaise
Nausea
Immunocompromised hosts (e.g. AIDS, immunodeficiencies) tend to present with a more chronic, severe gastrointestinal illness. These patients commonly present with:
Diffuse, foul-smelling diarrhea
Weight loss
Biliary disease(e.g. acalculous cholecystitis, sclerosing cholangitis)
The best, most cost effective initial diagnostic test is microscopic identification of the oocysts within the stool. This can be very difficult and may require multiple samples.
The most accurate diagnostic test for Cryptosporidium is an enzyme-linked immunosorbent assay (ELISA).
Cryptosporidium may result in complications such as:
Severe dehydration
Malabsorption
Weight loss
Acalculous cholecystitis
Cholangitis
Pancreatitis
Immunocompetent hosts respond well to fluid resuscitation and typically do not require pharmacological treatment. When therapy is required, the drug of choice in both children and adults is nitazoxanide.
The most important therapy for HIV-infected patients is supportive(e.g. fluid and electrolyte replacement) and initiation of HAART if not already begun. If this fails, nitazoxanide should be initiated.
Biliary Disorder
Primary Biliary Cholangitis
Primary biliary cholangitis (formerly primary biliary cirrhosis) (PBC) is an autoimmune disorder in which there is continuous destruction of the intrahepatic bile ducts, resulting in cholestasis and end stage liver disease.
PBC most commonly occurs in middle-aged females, and tends to have a greater prevalence in people of Northern European descent.
Pruritus and fatigue are the 2 most common presenting symptoms. Other symptoms include:
Vague musculoskeletal pain
Skin hyperpigmentation
Right upper quadrant tenderness
Xanthomas and xanthelasmas
A diagnosis of PBC is confirmed when two of the following three criteria are met:
Positive anti-mitochondrial antibodies (AMA) within the serum
Elevated alkaline phosphatase
Liver biopsy revealing destruction of the intrahepatic bile ducts
Other helpful diagnostic tests in diagnosing PBC include ultrasound, CT, and MRI.
Complications of primary biliary cholangitis (PBC) include:
Malabsorption
Cirrhosis
Hepatocellular carcinoma
Metabolic bone disease (e.g. osteopenia, osteoporosis)
Note that metabolic bone disease in patients with PBC may be due to the inhibition of osteoblast activity by a retained toxin rather than vitamin D malabsorption.
The only approved pharmacologic agent in the treatment of PBC is ursodeoxycholic acid. This drug improves liver function test abnormalities and may delay the progression of disease symptoms.
Other second line agents include corticosteroids, methotrexate, and colchicine. Antipruritic agents include antihistamines (first-line), cholestyramine and colestipol.
Liver transplantation is the only curative therapy for PBC.
Primary Sclerosing Cholangitis
Primary sclerosing cholangitis is a progressive disorder characterized by inflammation and fibrosis of the intrahepatic and extrahepatic biliary ducts, which results in cholestasis and cirrhosis of the liver.
The exact etiology of PSC is still unknown, but there are several factors shown to have an increased risk, such as:
Ulcerative colitis
Male gender (70%)
Positive antineutrophil cytoplasmic antibodies(possibly predominantly classical p-ANCA or atypical p-ANCA, but is debated in literature, and are of limited diagnostic value)
First-degree relatives with the disease
Patients with primary sclerosing cholangitis typically present with:
Fatigue
Pruritus
Jaundice
Right upper quadrant pain
Approximately 75-85% of patients with primary sclerosing cholangitis haveinflammatory bowel disease (in particular, ulcerative colitis), which means they may also present with bloody diarrhea and abdominal pain.
An endoscopic retrograde cholangiopancreatography (ERCP)is the best test in diagnosing primary sclerosing cholangitis.
ERCP will reveal dilations and strictures of the intrahepatic and extrahepatic ducts, often referred to as “beads on string.”
Common physical exam findings include:
Excoriations from pruritic skin
Jaundice
Hepatosplenomegaly
Weight loss
Laboratory studies may be helpful in detecting the degree of liver damage and the amount of cholestasis, such as:
Alkaline phosphatase (3-5x the normal)
ALT, AST
Bilirubin
Albumin
Perinuclear anti-neutrophil cytoplasmic antibodies (p-ANCA)
Primary sclerosing cholangitis can result in the following complications:
Cirrhosis
Portal hypertension
Cholangitis
Steatorrhea
Vitamin deficiencies
Cholangiocarcinoma
Death
Currently there are no proven pharmacologic therapies that slow the progression of PSC. Therapy is aimed at treating the complications and obtaining a liver transplant, which is the only proven treatment for PSC.
Endoscopic dilation of strictures has been shown to have significant improvements in lab values, but these benefits are only temporary and do not affect disease progression.
The use of ursodeoxycolic acid in conjunction with endoscopic dilation of strictures has been shown to improve liver function and survival for some patients.
Last updated