Stroke
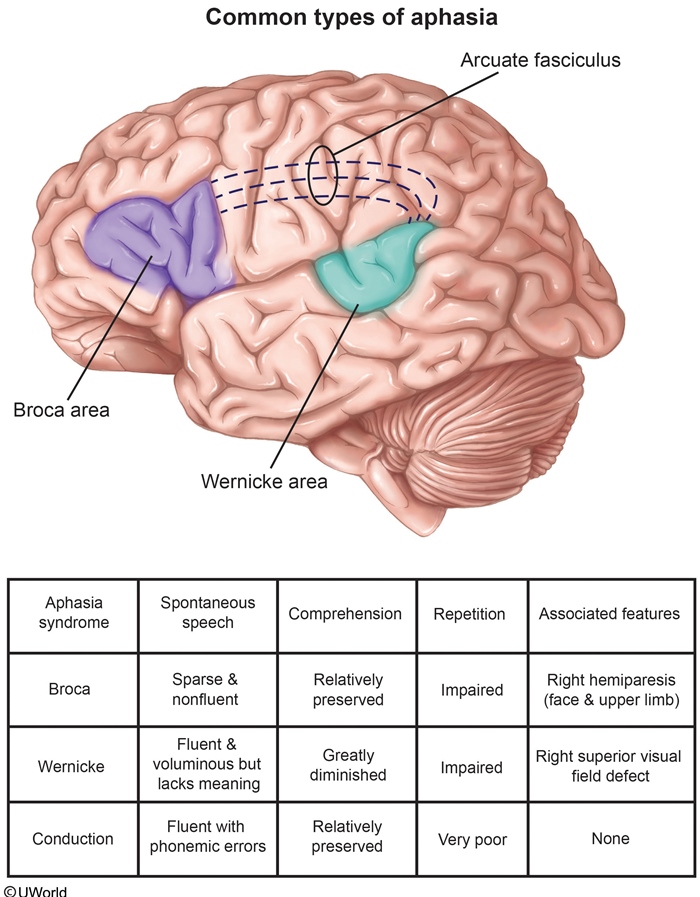
Aphasia
Broca's
Broca’s aphasia is an expressive aphasia, which is a loss of the ability to produce language.
Broca aphasia results from damage to Broca’s area (language motor area), which includes the following areas of the dominant hemisphere:
Inferior frontal gyrus
Dorsolateral frontal cortex
Anterior parietal cortex
Patients with Broca aphasia show the following symptoms:
Nonfluent - Production of few words or difficulty producing words
Good comprehension
Loss of oral coordination
Possible face and arm hemiparesis (from damage to the adjacent motor cortex)
Wernicke
Wernicke’s aphasia is a receptive aphasia, which is a loss of the ability to understand language.
Wernicke’s aphasia results from damage to Wernicke’s area (language understanding area), which includes the following areas of the brain:
Posterior superior temporal gyrus
Inferior parietal lobe
Patients with Wernicke’s aphasia show the following symptoms:
Fluent speech
Poor comprehension, which presents as word substitutions, meaningless words, or meaningless phrases.
Conductive
Conduction aphasia, also known as associative aphasia, is a loss of the ability to tie in language production and language understanding.
Conduction aphasia is the result of damage to the arcuate fasciculus, which connects Broca’s and Wernicke’s areas of the brain. This includes:
Supramarginal gyrus
Angular Gyrus
Symptoms of patients with conduction aphasia include:
Fluent speech
Difficulty in Repetition
Frequent word correction attempts
Word substitutions
Word-finding pauses
Global
Global aphasia is a complete loss of language expression, understanding, and association.
Global aphasia results from decreased blood flow through the middle cerebral artery (MCA), leading to massive infarcts of the left cerebral hemisphere, thereby damaging:
Broca’s area
Wernicke’s area
Arcuate fasciculus
Symptoms of patients with global aphasia include:
Nonfluent speech
Poor comprehension
Difficulty producing words
Limb ataxia, which is a result of damage to adjacent motor cortex
Stroke
2 types of strokes in general: ischemic, hemorrhagic
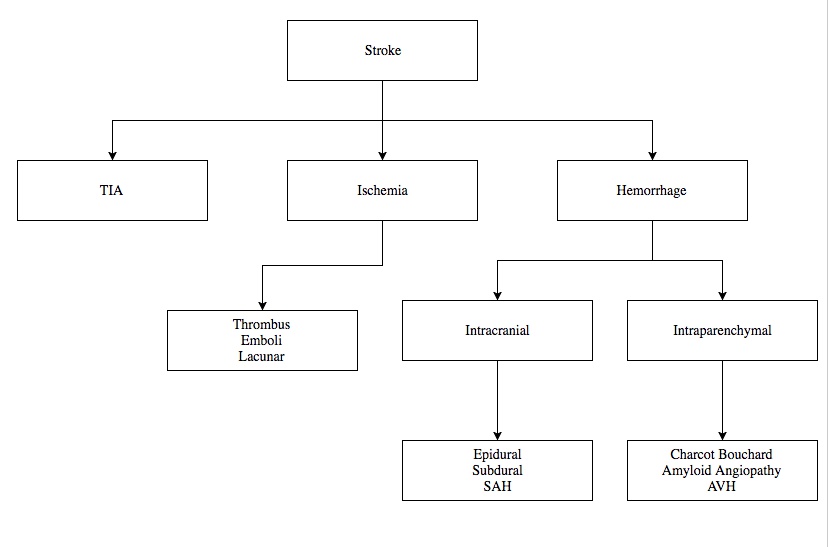
Ischemic
Ischemic stroke results when blood flow to the brain is limited, resulting in decreased oxygenation of brain parenchyma.
Ischemic stroke or cerebrovascular accident (CVA) is the leading cause of neurologic disability and third leading cause of death.
Thrombosis and emboli are the two mechanisms by which an ischemic stroke can occur. Can also be lacunar.
Embolic
Embolic stroke is the most common etiology of CVA, most commonly originating from:
Heart
Internal carotid artery
Aorta
Emboli that arise from blood clots in the peripheral veins.These patients will also likely have a patent foramen ovale.
Thrombotic
Thrombotic strokes occur due to atherosclerotic lesions within the carotid artery or the arteries of the brain.
Lacunar
Another form of ischemic stroke is a Lacunar stroke. Lacunar stroke is a small vessel thrombotic disease with narrowing of the arterial lumen due to thickening of the vessel wall.
Lacunar strokes are the cause of 20% of all strokes.
Lacunar infarcts affect the subcortical structures supplied by small branches of the Middle Cerebral Artery (MCA), circle of Willis, basilar and vertebral arteries.
Patients who have lacunar strokes typically have a history of hypertension.
Risks
Risk Factors for ischemic stroke include both lifestyle risk factors and comorbidities of the patient.
Life style risk factors:
Smoking
Diet
Vasoconstrictive drug use
Oral contraceptive use
Comorbidities associated with stroke:
Previous stroke or family history of stroke
Hypercoagulability
Hypertension
Diabetes
Atrial Fibrillation
Symptoms
Clinical features of CVA depend on the type of ischemic stroke and the location of the occlusion.
Embolic strokes are very rapid in onset and present with maximal deficits.
Thrombotic strokes can be rapid or stepwise in onset and the patient typically awakens from sleep with the deficits.
Complications of ischemic stroke include:
Progression of the neurologic deficits
Cerebral edema leading to mass effects
Hemorrhage
Seizures
Strokes that occur in the anterior cerebral artery (ACA) present with contralateral lower extremity motor and sensory deficiencies.
Strokes that occur in the middle cerebral artery present with contralateral hemiparesis (if proximal to small branch), hemisensory loss, aphasia, and apraxia. Symptoms are more common in the upper extremities than the lower extremities.
Strokes that occur in the posterior cerebral artery (PCA) most commonly present with hemianopia. In addition, infarction of the left PCA may present with:
Alexia without agraphia: can write, can't read
Anomic aphasia: word finding difficulty
Gerstmann syndrome: agraphia, acalculia, finger agnosia
And infarction of the right PCA may present with:
Prosopagnosia: can't recognize face
Spatial disorientation
Visual neglect
Strokes that occur within the vertebral or basilar arteries present with:
Ipsilateral ataxia
Ipsilateral diplopia
Ipsilateral dysphagia
Ipsilateral dysarthria
Vertigo
Lacunar strokes typically present as pure motor or pure sensory deficits.
Management
Careful observation for stroke progression and cerebral edema in patients with acute stroke is indicated for 24-36 hours. Patients should be monitored on a telemetry floor, and neurologic checks should be performed every 4 hours.
When diagnosing a CVA, a noncontrast CT scan is the first line imaging test to be ordered.
An infarct will not appear on CT scan for 24-48 hours, but this test is important to rule out intracranial hemorrhage and to identify patients who will benefit from thrombolytic therapy.
MRI of the brain is the most sensitive test to identify all early ischemic changes but is time consuming and should not be used in the acute setting.
Other important tests include
Labs to assess CBC, cardiac enzymes, electrolytes, BUN, creatinine, lipid profile, PTT, PT and INR.
Carotid duplex to assess for stenosis
ECG and echocardiogram to assess for atrial fibrillation or acute MI that may be a source of emboli
Magnetic Resonance Arteriogram (MRA) to identify stenosis of vessels within the head and neck.
Assessment of hypercoagulable states in those with a history of thombotic events
Treatment of ischemic stroke typically includes the use of intravenous recombinant tissue plasminogen activator (tPA, also called alteplase) if the drug can be administered within 3-4.5 hours of the onset of symptoms, and there are no contraindications to its use.
Exclusion criteria for tPA include
Significant head trauma or intracranial surgery
Previous intracranial hemorrhage
Intracranial neoplasm or arterial malformation
Current anticoagulant use
Platelet count less than 100,000
Uncontrolled hypertension
Age >80
Do not give tPA if the time of the stroke is unknown
If the patient cannot receive tPA, aspirin (160-325 mg/day) is best given within 24 hours. If a patient cannot take aspirin, clopidogrel should be used.
If the patient has received tPA, wait 24 hours before beginning an aspirin regimen.
In an ischemic stroke patient, it is important to allow for permissive hypertension. The increased blood pressure will help maintain perfusion of the ischemic tissue. However, if the patient has systolic BP >220 mmHg or diastolic >120 mmHg they should be treated with IV labetalol or nicardipine.
Carotid endarterectomy is indicated in symptomatic patients with ≥70% carotid artery stenosis or asymptomatic patients with ≥80% stenosis.
Other long term preventative measures for future ischemic stroke include:
Aspirin or clopidogrel use if stroke is secondary to small vessel disease or thrombosis
Anticoagulation in patients with atrial fibrillation or hypercoagulable disorders
Management of comorbidities (e.g. hypercholesterolemia, diabetes, hypertension)
Parenchymal Hemorrhage
Pathogenesis
Parenchymal hemorrhage, also known as intracerebral hemorrhage, is bleeding within the brain tissue.
Parenchymal hemorrhage has multiple mechanisms that lead to brain injury, including:
Direct injury due to an expanding blood clot
Mass effect leading to increased intracranial pressure
Ischemic injury
Parenchymal hemorrhage is the second most common cause of stroke, second only to ischemic stroke in frequency.
Small vessel vasculopathy secondary to chronic hypertension is the most common cause of parenchymal hemorrhage. The underlying pathology is a combination of microatheroma formation and lipohyalinosis that ultimately leads to thrombotic small-vessel occlusion.
Cerebral amyloid angiopathy is the most common cause of nontraumatic lobar parenchymal hemorrhage in elderly patients.
Intracranial vascular anomalies (e.g. arteriovenous malformations) are the most common cause of parenchymal hemorrhage in children.
Exposure to vascular perfusion injuries have also been associated with IVH. Examples of such events are: (1) hypoxic or ischemic episodes, (2) hypotension, (3) reperfusion of damaged vessels, (4) increased venous pressure, and (5) abrupt changes in cerebral flow.
Parenchymal hemorrhages due to hypertension most commonly occur in the basal ganglia (especially the putamen), thalamus, and pons.
In contrast, parenchymal hemorrhages due to cerebral amyloid angiopathy most commonly occur in the parietal and occipital lobes.
Symptoms
Patients with parenchymal hemorrhage present with focal neurologic symptoms (e.g. hemiplegia, homonymous hemianopsia) that are followed by manifestations of increased intracranial pressure (e.g. headache, vomiting). These manifestations intensify gradually over time.
In contrast, patients with subarachnoid hemorrhage present abruptly with an intense headache without focal neurologic symptoms.
Seizures can occur in the days following a parenchymal hemorrhage; this is especially common in patients with superficial (e.g. lobar) hemorrhages.
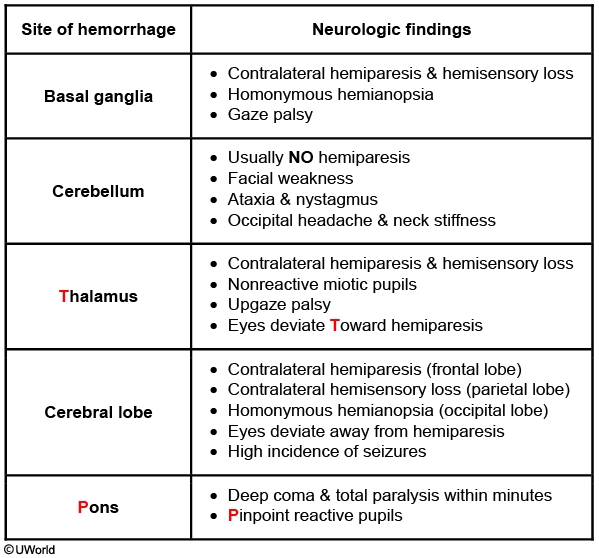
Cerebellar Symptoms
Typical features of cerebellar hemorrhage include occipital headache (may radiate to neck/shoulders), neck stiffness (due to extension of blood into the 4th ventricle), nausea/vomiting, and nystagmus. Patients may also have ipsilateral hemiataxia of the trunk (cerebellar vermis) and/or limbs (cerebellar hemispheres) as the corticopontocerebellar fibers decussate twice. Hemiparesis and sensory loss are usually absent, but extension of the bleed to the brainstem can cause cranial neuropathies (eg, ipsilateral facial nerve palsy). Further hematoma expansion may lead to brainstem compression resulting in stupor, coma, and ultimately death. Early diagnosis with noncontrast head CT scan is crucial as emergency decompression may be life-saving, especially if the hemorrhage is >3 cm.
Diagnosis and Management
Noncontrast head CT scan is most widely used to diagnose parenchymal hemorrhage.
Magnetic resonance angiography or CT angiography can be used to localize the site of bleeding.
MRI may be more useful in diagnosing micro hemorrhages within the brain parenchyma.
Treatment of parenchymal hemorrhage is supportive care. Focus is made on:
Maintaining normal intracranial pressure
Maintaining normal blood pressure
Using anticonvulsants to control seizure activity
Surgical decompression for large hemorrhages
Surgical repair of any arteriovenous malformations or aneurysms may be required.
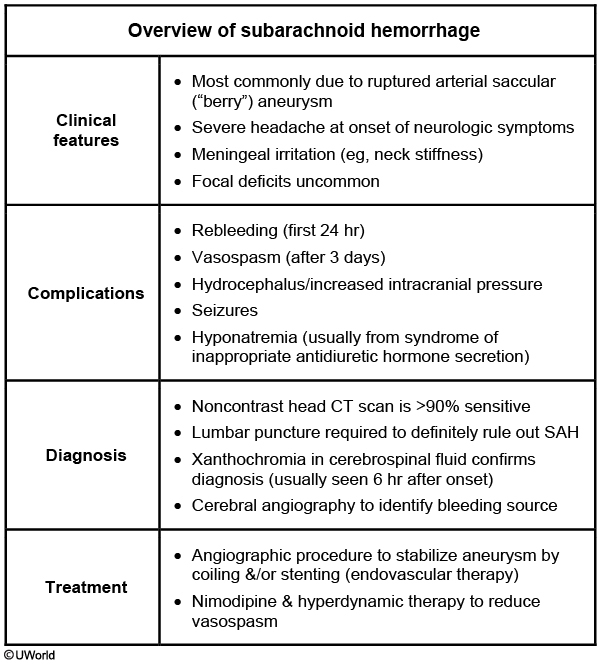
Subarachnoid Hemorrhage
Subarachnoid Hemorrhage is a brain bleed that occurs below the arachnoid layer of the meninges and is most commonly due to the rupture of a berry aneurysm.
Other causes include trauma to the circle of Willis, or an arteriovenous malformation.
Risk factors for subarachnoid hemorrhage include:
Hypertension (most common)
Autosomal dominant polycystic kidney disease (ADPKD)
Bleeding disorders or use of a blood thinner
Ehlers-Danlos syndrome
Smoking (most important preventable risk factor)
Common sites for the occurrence of a subarachnoid hemorrhage include:
Junction of the anterior communicating artery and anterior cerebral artery
Junction of the posterior communicating artery and the internal carotid artery
Bifurcation of the middle cerebral artery
Symptoms
Many patients who have a subarachnoid hemorrhage will present with the sudden onset of a severely painful headache (“worst headache of my life”). Thunderclap headache
The onset of the headache may or may not be associated with a brief loss of consciousness, nausea or vomiting, and meningismus.
Diagnosis
Diagnosis of a subarachnoid hemorrhage is made by a noncontrast CT scan.
A lumbar puncture is performed to diagnose subarachnoid hemorrhage (SAH) if there is a strong suspicion of SAH and the CT scan is negative. The presence of blood in the cerebrospinal fluid or xanthochromia (yellow tint of the cerebrospinal fluid) is indicative of SAH.
The patient must be assessed for papilledema before performing the lumbar puncture so as not to cause herniation. If papilledema is present, repeat the CT scan first.
Treatment of a subarachnoid hemorrhage is surgical clipping and coiling.
Cerebral angiogram is the gold standard test for determining the location of bleeding in patients with a SAH.
The patient must be medically managed to reduce the risk of rebleeding. This includes managing blood pressure, using anti-seizure medications to prevent seizures, and stool softeners or laxatives to prevent straining during bowel movement
Symptomatic vasospasm and cerebral infarction are important contributors to unfavorable outcomes after SAH. It typically begins after day three post hemorrhage, reaching a peak at days seven to eight. Nimodipine may mitigate this complication.
Hyponatremia is a potential complication due to "cerebral salt-wasting syndrome" or SIADH.,
Transient Ischemic Attack
Transient ischemic attack (TIA) is defined as an acute episode of neurologic dysfunction caused by focal brain, spinal cord, or retinal ischemia, without acute infarction. A TIA becomes an ischemic stroke only when infarction of CNS tissue occurs, and not necessarily a time course of greater than 24 hours.
Transient ischemic attack is caused by a temporary disruption of blood supply to the brain from underlying conditions such as aortic stenosis, vascular spasm, atherosclerosis, and emboli (the same conditions that cause strokes).
Risk factors for a TIA include:
Age over 55 years
Hypertension
Diabetes mellitus
Coronary artery disease
Smoking
Hyperlipidemia
Hypercoagulable states
Having a TIA puts a patient at risk for experiencing a stroke in the future.
Symptoms of a TIA present very much like that of a stroke, with a patient experiencing weakness, paralysis, numbness, loss of coordination, vision abnormalities, slurred speech, and mental confusion.
The symptoms appear suddenly, but typically abate within one day, with many cases resolving within an hour.
Physical exam may reveal carotid bruits or harsh systolic murmurs indicative of carotid atherosclerosis and aortic stenosis respectively. Physical exam may also reveal an irregularly irregular rhythm that is indicative of atrial fibrillation.
Diagnosis
Diagnosis can be based on a thorough history and physical exam accompanied by CT or MRI findings. MRI is more likely to identify infarction of tissue.
Ultrasound can be useful for determining possible sources of atherosclerosis.
Echocardiography can be useful to determine heart defects as an underlying cause of emboli.
Magnetic resonance angiography (MRA), computed tomography angiography (CTA), carotid duplex ultrasonography (CDUS), and transcranial doppler ultrasonography (TCD) can be used to identify if there is an obstructive lesion in a larger artery supplying the CNS.
Treatment consists of addressing the underlying conditions leading to ischemia.
Patients with TIA due to atherosclerosis should be given antiplatelet (e.g. aspirin or clopidogrel) andstatin therapy.
Patients with TIA due to severe carotid stenosis (i.e. 70 to 99% occlusion, symptomatic disease) should undergo carotid endarterectomy to increase blood flow to the brain.
Patients with TIA due to aortic stenosis should be offered beta blockers, valvuloplasty or valve replacement.

if resolve or outside 4 hour window: use aspirin
Last updated