08 Cardiology
CABG
A coronary artery bypass graft (CABG) utilizes arteries or veins from elsewhere in the patient's body to bypass atherosclerotic stenosis and improve coronary blood flow. The 2 primary indications for coronary artery bypass graft (CABG) are patients with:
Left main coronary stenosis of 50%
3-vessel disease
Percutaneous coronary intervention is indicated in patients with single or two vessel disease and normal LV function.
Grafts used in coronary artery bypass surgery include the internal thoracic artery and saphenous vein, among others. Internal thoracic arteries are more commonly used as they have been shown to have the best long term patency.
The four most common noncardiac complications of CABG are:
Bleeding
Stroke, or other neurologic problems
Infection: mediastinitis
Acute kidney injury
Mediastinitis
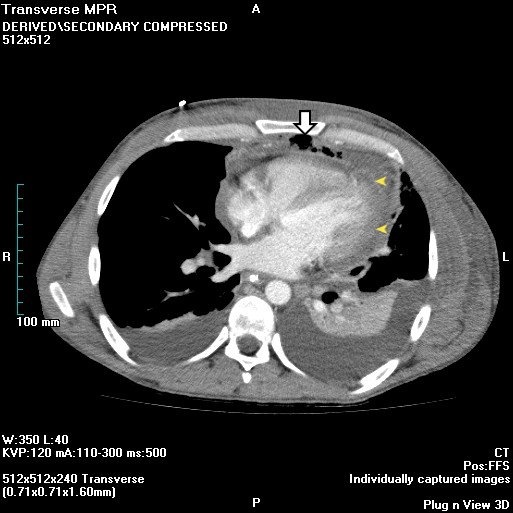
This patient presents with findings consistent with likely acute mediastinitis, a possible complication of cardiac surgery that is usually due to intraoperative wound contamination. Mediastinitis can complicate up to 5% of sternotomies. Patients typically present post-operatively (usually within 14 days) with fever, tachycardia, chest pain, leukocytosis, and sternal wound drainage or purulent discharge (as in this patient). Hamman’s sign is a crunching sound heard with a stethoscope over the precordium during systole and is suggestive of acute mediastinitis.
Chest x-ray usually shows a widened mediastinum in non-postoperative mediastinitis, but this can also be seen in postoperative mediastinitis after cardiac surgery. Chest radiograph findings include pneumomediastinum and/or air-fluid levels within the mediastinum. A CT scan can also support the diagnosis by demonstrating dehiscence of the sternum and stranding, fluid and air pockets within the anterior mediastinum. The diagnosis is usually clinically made and confirmed during surgery when pus is noted in the mediastinum. Postoperative mediastinitis requires drainage, surgical debridement with immediate closure, and prolonged antibiotic therapy. Antibiotics alone do not appropriately treat mediastinitis. Acute mediastinitis has a mortality rate of 10%–50%, even with appropriate treatment.
AFIB
Atrial fibrillation commonly (up to 15%–40%) occurs within a few days after CABG and is usually self-limited, with resolution in less than 24 hours. Rate control with beta-blockers or amiodarone is best. Anticoagulation and/or cardioversion is reserved for patients with atrial fibrillation lasting >24 hours after CABG. Since this patient requires immediate surgery anticoagulation should not be given.
Catherterization
Hematoma
This patient's presentation (recent cardiac catheterization, anticoagulation with heparin, sudden onset of hypotension, tachycardia, flat neck veins, and back pain) is consistent with retroperitoneal hematoma due to bleeding from the arterial access site (with retroperitoneal extension). Local vascular complications at the catheter insertion site - bleeding, hematoma (localized or with retroperitoneal extension), arterial dissection, acute thrombosis, pseudoaneurysm, or arteriovenous fistula formation - are the most common complications of cardiac catheterization. Most hemorrhage or hematoma formation occurs within 12 hours of catheterization.
Patients with hematoma present with localized discomfort and/or swelling of the soft tissue. If the arterial puncture site is above the inguinal ligament, the hematoma can extend into the retroperitoneal space, even with minimal visible localized hematoma, and present with sudden hemodynamic instability and ipsilateral flank or back pain. Diagnosis is confirmed with non-contrast CT scan of abdomen and pelvis or abdominal ultrasound. Treatment is usually supportive, with intensive monitoring, bed rest, and intravenous fluids or blood transfusion. Surgical repair of hematomas or retroperitoneal hemorrhage is rarely required.
Prosthetic Valves
The two categories of valve replacement are tissue and mechanical.
Tissue valves are indicated in patients:
Older than 65
Who do not want anticoagulation
Women of childbearing age
Mechanical valves are indicated in patients:
Younger than 65
That have no contraindications to anticoagulants
Who are reliable to take anticoagulants
The St. Jude mechanical valve is the most commonly used valve in aortic and mitral valve replacement due to its double-disk tilting, hemodynamics, and low thrombogenicity.
The major risk associated with mechanical valves is the need for lifelong anticoagulation.
The major risk associated with a tissue valve is structural deterioration of the valve requiring 30% of patients in need of replacement in 10 years and 50% in 15 years.
Valve obstruction can result from thrombosis due to:
Poor anticoagulation
Formation of a fibrous tissue ingrowth called a pannus (less common)
Vegetations due to poor antibiotic prophylaxis
Non-structural dysfunction is a general term for valve complications that arise and include:
Inappropriate sizing
Hemolysis
Tissue or suture entrapment
Paravalvular leak
Operated prosthetic valvular endocarditis can be early (<60 days following implantation) or late. Early prosthetic valve endocarditis is when the valve is seeded intraoperatively or via catheters, cannulas, or wound infections.
Organisms involved in early prosthetic valve endocarditis include
Staphylococcus aureus
Staphylococcus epidermidis
Gram-negative bacteria
HACEK organisms
Haemophilus
Aggregatibacter (formerly Actinobacillus)
Cardiobacterium
Eikenella
Kingella
Pseudomonas
Late prosthetic valve endocarditis is typically due to noncardiac sepsis arising from dental, gastrointestinal, or genitourinary tracts.
Organisms involved in late prosthetic valve endocarditis include Streptococcus spp. and Staphylococcus spp. with viridans streptococci being the most common.
Aortic Stenosis
Aortic stenosis is narrowing of the aortic valve due to calcification of the valve leaflets or valvular damage.
Causes
The most common cause of aortic stenosis is calcification of otherwise normal valve leaflets with age; the prevalence increases rapidly in the 7th decade of life.
Aortic stenosis commonly occurs at an early age in patients with congenital bicuspid aortic valves.
Aortic stenosis can also result from rheumatic fever. Though rheumatic fever is most strongly associated with mitral stenosis, other valves may also be affected.
Symptoms
Advanced aortic stenosis is classically associated with syncope, angina, and heart failure. Disease may be asymptomatic up to this point.
The left ventricle grows progressively hypertrophied as the myocardium contracts against the mounting afterload. The myocardium outgrows its blood supply, leading to ischemia that results in progressive chest pain, dyspnea, and pulmonary congestion.
Aortic stenosis causes a systolic crescendo-decrescendo murmur heard loudest in the second intercostal space at the right sternal border, which often radiates to the carotid arteries or can be auscultated at the clavicles.
The murmur increases in intensity with maneuvers that increase preload (e.g. leg-raising) and decreases in intensity with maneuvers that decrease preload (e.g. Valsalva) or increase afterload (e.g. isometric hand-squeezing).
Aortic stenosis is associated with an S4 heart sound.
Physical exam may reveal pulsus parvus et tardus (weak peripheral pulses with a maximal intensity peak that is late relative to the heartbeat) due to the slowed emptying of left ventricle to the systemic circulation.
Diagnosis
Echocardiography confirms the physical findings by demonstrating a narrowed valve area with increased transvalvular pressure gradient and possible left atrial enlargement.
Cardiac catheterization is indicated to delineate the severity of disease when a noninvasive test proves non-diagnostic, or when there is a discrepancy between physical and echocardiographic findings.
Chest X-ray is usually normal but in cases of more severe and progressed disease may demonstrate a rounding of the left ventricular apex consistent with left ventricular hypertrophy. Note that valvular calcifications are not necessarily visible on chest radiograph, but may still be present.
Electrocardogram (ECG) will often show left ventricular hypertrophy (LVH), left atrial enlargement, and left bundle branch block (LBBB). Severe dilation of the left atrium can result in atrial fibrillation in late disease. Note that these findings are nonspecific.
Once symptomatic, the progression of aortic stenosis can be rapid, and patients are at increasing risk for sudden cardiac death.
The presentation of aortic stenosis has prognostic value. In order of worsening prognosis, this includes:
Angina
Syncope
Heart failure
Severely calcified aortic valves can shred von Willebrand factor multimers, leading to a bleeding diathesis that classically presents with gastrointestinal bleeding from angiodysplasia. (This is sometimes referred to as Heyde's syndrome.)
Though it more commonly occurs with prosthetic valves, mechanical shearing of red blood cells across the damaged calcific valve may lead to an intravascular hemolytic anemia. Supportive findings include schistocytes on peripheral smear, elevated lactate dehydrogenase (LDH), low haptoglobin, hemoglobinuria, and a normal platelet count in a patient with diagnosed aortic stenosis.
Management
Asymptomatic aortic stenosis is managed medically until surgical valve replacement is indicated and in patients who are not good surgical candidates.
Medical management is used in asymptomatic patients and symptomatic patients who are not surgical candidates. It consists of:
Medication to control hypertension (usually diuretic and ACE inhibitor/ARB)
Avoidance of vigorous physical exercise
Avoidance of venodilators (e.g. nitrates, phosphodiesterase inhibitors) and negative inotropes (e.g. calcium channel blockers/beta blockers) as these lessen preload
Note: Medications which affect loading conditions should be used with caution since patients with severely symptomatic aortic stenosis have a small therapeutic window.
Aortic valve replacement is the only definitive treatment for aortic stenosis. Indications for valve replacement according to the ACC/AHA guidelines include:
Symptomatic stenosis, as indicated by the presence of angina, syncope, or heart failure (CHF);
Severe stenosis in asymptomatic patients who are undergoing CABG, surgery or replacement of an additional valve, or of the aorta;
Severe stenosis in asymptomatic patients with systolic failure (ejection fraction less than 50%).
Severe stenosis is defined as a valve cross-sectional area less than 1.0 cm2, mean gradient greater than 40 mm Hg, or jet velocity greater than 4.0 m/s.
Intra-aortic balloon pump (IABP) is used for stabilization and bridge to surgery.
AVM
Arteriovenous malformations (AVMs) are congenital or acquired aberrant connections between arterial and venous circulations, which bypass the normal physiological pressure drop-off created by capillary beds.
AVMs involving the larger more proximal vasculature may result in a significant reduction in systemic vascular resistance (SVR).
Penetrating traumatic injuries with associated vascular damage may result in chronic AVMs.
Superficial AVMs can present as palpable, warm, pulsatile masses. Deeper AVMs may be asymptomatic or may produce symptoms secondary to mass effect and local ischemia as well as reduced SVR.
A persistently reduced SVR can eventually progress to high-output cardiac failure. Seizure and hemorrhagic stroke are two consequences for AVM in brain.
Large, symptomatic AVMs as well as AVMs located in the brain and bowel should be surgically removed or sclerosed.
Hypertensive Crisis
Hypertensive urgency is defined as a systolic blood pressure (SBP) >180 mm Hg or a diastolic blood pressure (DBP) >120 mm Hg without physical evidence of target organ damage.
Patients with hypertensive urgency present with non-progressive symptoms including headache, shortness of breath, epistaxis, or pedal edema.
Hypertensive emergency in non-pregnant patients is defined as the presence of end-organ damage (cardiac, renal, central nervous system, etc.) which is due to severely elevated blood pressure. Generally this occurs when DBP > 120 mm Hg, but may occur at lower pressures if there has been a rapid increase above baseline.
Though patients suffering from hypertensive emergencies may be asymptomatic, by definition end-organ damage is present. This may include:
Acute myocardial infarction
Hypertensive encephalopathy/retinopathy
Intracranial hemorrhage
Dissecting aneurysm
Acute renal failure
Flash pulmonary edema
SBP > 159 mm Hg or DBP > 109 mm Hg that persists for at least 15 minutes in a pregnant female is considered a hypertensive emergency, regardless of the presence or absence of additional symptoms, signs, or laboratory findings, and should be managed accordingly.
Most hypertensive crises are due to an acute exacerbation of underlying hypertension, frequently brought on by noncompliance with antihypertensive therapy. Additional causes include:
Medication side effect: oral contraceptives, attention deficit hyperactivity disorder (ADHD) drugs, nonsteroidal anti-inflammatory agents (NSAIDs), linezolid, and monoamine oxidase inhibitors (MAOIs)
Recreational drug use: cocaine, amphetamines, and phencyclidine (PCP, angel dust)
Pregnancy: pre-eclampsia/eclampsia, HELLP syndrome
Any cause of secondary hypertension (recall the DRAPE mnemonic)
Malignant hypertension is a subset of a hypertensive emergency marked by an accelerated course of rapidly rising BP and the presence of papilledema on physical exam.
Renal pathological changes associated with malignant hypertension include a flea bitten kidney (multiple hemorrhages due to ruptured arterioles in a large swollen kidney).
Management
Initial evaluation of a hypertensive crisis should include:
Serum electrolytes to rule out hypokalemia or hypomagnesemia
Serum BUN and creatinine to assess renal function
Liver function tests (AST, ALT, alkaline phosphatase, PT/INR)
Complete blood count with peripheral smear
Urinalysis with microscopy
Electrocardiogram
Chest x-ray if the patient complains of chest pain
Non-contrast CT in patients with neurological symptoms or complaining of a headache
Urgency treatment: oral captopril, furosemide, or clonidine.
The treatment of hypertensive emergency requires three steps:
Immediate admission to an intensive care unit (ICU)
Parenteral (IV) administration of a titratable anti-hypertensive agent
Continuous monitoring of blood pressure, neurological status, and urine output
For most hypertensive emergencies, mean arterial pressure should be reduced by no more than 25% within the first 24 hours. This is usually accomplished by a reduction of 10% during the first hour and an additional 15% over the next 23 hours.
The most appropriate IV agent(s) to treat a hypertensive emergency are dictated by the presentation:
Hypertensive encephalopathy: labetalol
Pregnancy: hydralazine
Aortic dissection: 2 IV agents(e.g. labetalol + esmolol)
(Please refer to the chart for a more complete approach including more conditions and treatment options.)
Prolonged infusion with nitroprusside can lead to cyanide toxicity (nitroprusside contains 5 cyanide groups per molecule), particularly in pediatric patients and patients with chronic renal failure. Patients present with altered mental status and lactic acidosis.
Secondary Hypertension
Causes
Secondary hypertension is hypertension due to an identifiable underlying etiology. Often these patients have a history of hypertension refractory to the maximal dosage of several drugs.
The causes of secondary hypertension can be organized into several categories:
Drug- or medication-related
Renal failure (renal parenchymal disease)--most common non-medication-related etiology
Anatomic (vascular) anomalies: aortic coarctation, renal artery stenosis (fibrodysplasia, renal artery stenosis/atherosclerosis)
Pheochromocytoma
Endocrinopathies: hyperaldosteronism (Conn syndrome), hypercortisolism (Cushing syndrome), hyperparathyroidism, hyperthyroidism
Using the mnemonic DRAPE may help you recall the various etiologies of secondary hypertension when deciding on the next best step in management.
Renal artery stenosis is more common in female patients less than 25 years of age (fibromuscular dysplasia) and in patients over the age of 50 (atherosclerosis).
The presence of a persistent abdominal bruit that lateralizes to one side is highly suggestive of renovascular hypertension (sensitivity of 40%, specificity of 99%).
An acute increase in serum creatinine by ≥30% following initiation of an ACE inhibitor or ARB in a patient that is not hypovolemic is highly suggestive of renovascular hypertension.
While fibromuscular dysplasia most commonly involves the renal arteries, it can also involve the carotid and vertebral arteries. Thus, patients can present with headache, pulsatile tinnitus, and neck pain as well as manifestations of CNS ischemia (e.g. amaurosis fugax, transient ischemic attack).
Combination oral contraceptive pills (OCPs) are more likely to cause hypertension in women over 35 years of age, obese women, and in patients with a history of long-term OCP use.
Pheochromocytomas occur more commonly in younger patients with a history of endocrine tumors.
Aortic coarctation is more common in patients with Turner syndrome, especially on test questions.
Newly diagnosed hypertension deserves a search for contributing factors: history should include review of current medications (oral contraceptives, NSAIDs, antidepressants, glucocorticoids, decongestants, stimulants), recreational drug use, and questions about the signs and symptoms that suggest an underlying condition (Cushing disease, pheochromocytoma, obstructive sleep apnea, etc.).
To remember some common medications which cause elevations in blood pressure, it may help to recall the mnemonic GONADS:
Glucocorticoids
OCPs (oral contraceptive pills)
NSAIDs (non-steroidal anti-inflammatory agents)
Antidepressants (particularly SNRIs)
Decongestants (pseudoephedrine)
Stimulants (methylphenidate, amphetamines)
Secondary hypertension treatment depends on etiology. Cause
Treatment
Renal parenchymal disease
ACE inhibitor or ARB to delay progression
Renal artery stenosis
Angioplasty, stent placement; ACE inhibitor or ARB if one-sided (contraindicated in bilateral renal artery stenosis because renal perfusion and GFR will decrease)
Combination oral contraceptives
Stop OCP, change to progestin-only pill or intramuscular medroxyprogesterone
Pheochromocytoma
Control hypertension pharmacologically (phenoxybenzamine x 10 to 14 days, then add propranolol 1 to 2 days before surgery); then adrenalectomyafter 10 to 14 days of alpha blockade
Primary hyperaldosteronism (Conn syndrome)
Aldosterone antagonists (spironolactone, eplerenone) and surgical resection
Excess glucocorticoids (Cushing syndrome)
Treat underlying cause and reduce exogenous steroids
Aortic coarctation
Surgical correction
Hyperparathyroidism/hypercalcemia
Hydration
Left CHF
Causes
Left congestive heart failure (CHF) is evaluated and treated on the basis of whether the ejection fraction (EF) is preserved or reduced:
CHF with reduced EF occurs when insufficient cardiac output is due to reduced ventricular contraction. This is sometimes referred to as systolic dysfunction, and is the more common pathophysiological type.
CHF with preserved EF occurs when insufficient cardiac output is due to reduced ventricular compliance. This is sometimes referred to as diastolic dysfunction.
Systolic dysfunction occurs when chronic volume overload leads to eccentric hypertrophy (i.e. sarcomeres added in series), and is most often a sequela of cardiac ischemia and coronary artery disease (CAD). You can think of this as the heart's attempt to accommodate a chronically escalating preload through eccentric remodeling.
Diastolic dysfunction occurs when chronic pressure overload leads to concentric hypertrophy (i.e. sarcomeres added in parallel), and is most often a sequela of poorly managed hypertension. You can think of this as the heart's attempt to accommodate a chronically escalating afterload through concentric remodeling.
Symptoms
Left-sided heart failure generally presents with symptoms of pulmonary congestion and fluid overload:
Dyspnea on exertion
Orthopnea
Paroxysmal nocturnal dyspnea
Nocturia
Note that the clinical presentation of systolic and diastolic heart failure are indistinguishable from each other, as they both result from a decrease in forward blood flow.
Dyspnea on exertion is caused by interstitial fluid in the lung stimulating juxtacapillary receptors (J receptors), innervated by the vagus nerve, causing rapid shallow breathing.
Orthopnea refers to shortness of breath that occurs when laying flat that results from an increase in venous return. The severity of orthopnea is assessed by asking the patient the number of pillows on which they sleep to avoid breathlessness.
Paroxysmal nocturnal dyspnea (PND) refers to severe breathlessness that awakens a patient from sleep. Gradual reabsorption of fluid from the interstitium of the lower extremities into the vascular compartment leads to increased venous return, which worsens pulmonary congestion. While orthopnea is a direct manifestation of cardiac insufficiency, PND results from the fluid-avid state that serves as a compensatory mechanism to try to accommodate diminishing forward cardiac output by increasing preload.
Fluid overload occurs as the decreased cardiac output causes an activation of renin-angiotensin-aldosterone system with retention of salt and water.
Hemoptysis may occur in left heart failure due to the rupture of engorged bronchial veins.
Brick-red sputum results from an increased pressure in the alveolar capillaries that causes red cells to leak out. Alveolar macrophages ingest red blood cells. These hemosiderin-laden macrophages are called heart failure cells.
Diagnosis
Diagnosis of CHF is based primarily on clinical history and physical exam, which should include initial and serial measurements of weight as a proxy for determining volume status.
An S3 "Kentucky" gallop is classically associated with systolic dysfunction.
An S4 "Tennessee" gallop is classically associated with diastolic dysfunction.
Cardiogenic pulmonary edema may be detected as rales over both lung fields most prominent at the bases, and may be accompanied by expiratory wheezing.
Echocardiography or catheterization in systolic heart failure demonstrates reduced EF, increased end-diastolic volume (EDV), and elevated end-diastolic pressure (EDP, filling pressure).
Echocardiography or catheterization in diastolic heart failure demonstrates low-to-normal EF, decreased EDV, elevated filling pressure.
Brain natriuretic peptide (BNP)is commonly used to clarify acute CHF exacerbations, but serial measurements are not generally recommended to monitor treatment response.
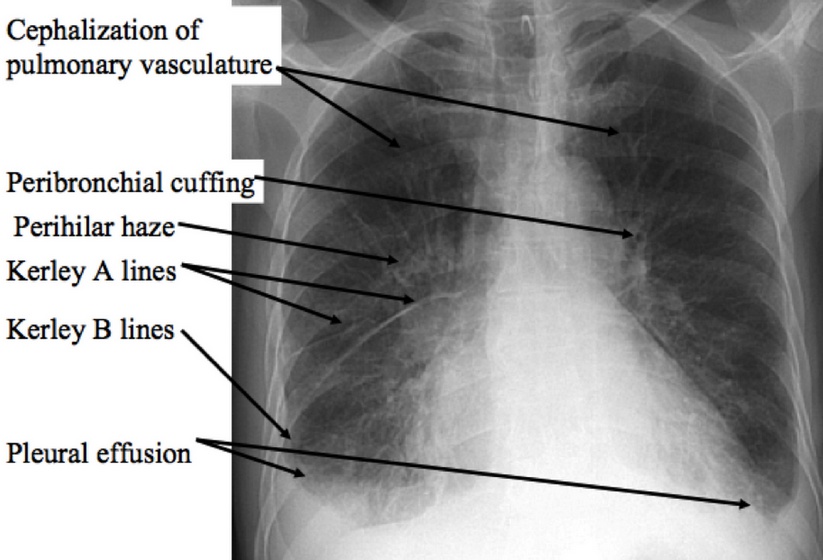
Chest x-ray findings include:
Kerley B lines (thin pulmonary opacities caused by fluid in the interstitium of the lung)
Enlarged cardiac silhouette
Peribronchial cuffing (excess fluid in the small airway passages of the lung causes localized patches of atelectasis)
Cephalization of the pulmonary vasculature (antigravitational redistribution of the pulmonary blood flow due in part to increased pulmonary vascular resistance and pulmonary hypertension)
Management
Acutely decompensated CHF exacerbations are most appropriately treated with LMNOP:
Lasix (furosemide) or another loop diuretic to reduce volume overload
Morphine to reduce anxiety and relieve chest pain
Nitroglycerin
Oxygen (supplemental)
Positioning (seated upright)
Initial treatment for heart failure begins with asymptomatic, high-risk patients who have yet to develop structural heart disease (ACCF/AHA Class A). This consists of controlling hypertension, lipid disorders, and diabetes mellitus (DM), in addition to lifestyle modifications targeted at reducing obesity, smoking cessation, and limiting alcohol consumption.
Long term treatment is based on symptomatic measures as well as primary and secondary prevention of sudden cardiac death. For testing purposes, only the following pharmacologic interventions have been shown to improve survival in patients with heart failure with reduced ejection fraction (i.e. systolic failure):
Beta-blockers (carvedilol, metoprolol succinate, and bisoprolol ONLY)
Angiotensin-converting enzyme inhibitors (ACEI) or angiotensin II receptor blockers (ARBs)
A ldosterone antagonists (spironolactone) for patients NYHA Class III & IV
Hydralazine + isosorbide dinitrate, in addition to standard therapy with the above drugs for African-Americans
The combination of hydralazine + isosorbide dinitrate is recommended as a replacement for ACEIs or ARBs in patients who are unable to tolerate any drugs in either class.
Diuretics are used for symptomatic control and fluid overload management. Lasix (furosemide) is most often used for its potency. Metolazone may be added to furosemide to increase potency and diuresis. Loop diuretics have the added benefit of opposing the hyperkalemia caused by beta-blockers, ACE inhibitors, ARBs, and aldosterone antagonists.
Inotropes such as digoxin provide symptomatic relief and decrease hospitalizations, but have no overall effect on mortality.
Calcium channel blockers have equivocal effects on survival and arecurrently not recommended for treatment of CHF.
Temporal Arteritis
Temporal (giant cell) arteritis is a chronic vasculitis that affects medium- and large-sized arteries with a proclivity for the cranial branches of the arteries that originate from the aortic arch.
It is characterized pathologically by the presence of subacute granulomatous inflammation.
The inflammation is segmental, with intervening segments of normal and inflamed vessel wall.
Temporal arteritis is more common in patients over the age of 50, and occurs more frequently in women.
Nearly half of the patients with temporal arteritis also have polymyalgia rheumatica.
Symptoms of temporal arteritis include:
Severe headache of sudden onset
Exquisite temporal tenderness
Jaw pain with chewing due to claudication
Scalp pain
New monocular blindness
Fever
Myalgias and arthralgia (may be due to polymyalgia rheumatica)
Without treatment, the monocular blindness of temporal arteritis can become permanent.
The gold standard for diagnosing temporal arteritis is temporal artery biopsy, though this should never delay treatment. Biopsy will demonstrate granulomatous inflammation of the media and adventitia with a lymphocytic infiltrate. Since the lesions are segmental, a relatively long (minimum 2 cm) segment of temporal artery is required for diagnosis. Note that a negative biopsy does not rule out the disease.
Patients with temporal arteritis will usually have a markedly elevated erythrocyte sedimentation rate (ESR) and C-reactive protein (CRP). Suspicion for temporal arteritis is often considered one of the only (if not the only) true indication for ordering an ESR because the gold standard for diagnosis (i.e. biopsy) is time-consuming, and an appropriately elevated ESR in a patient with the defining symptomatology has a high positive predictive value for a positive biopsy.
Physical examination will reveal a prominent temporal artery with beading, tenderness to palpation, and diminished or absent pulse.
Doppler ultrasound may show stenosis or occlusion of the involved artery, but is highly operator-dependent and should never replace biopsy as a means of making the diagnosis.
Fundoscopic examination in patients presenting with acute visual loss secondary to temporal arteritis may reveal cotton wool spots; a swollen, pale disc with blurred margins; and a cherry-red macula.
Examination of patients presenting later with permanent visual loss may demonstrate optic atrophy; a flat, pale disc; and a Marcus-Gunn pupil with a positive swinging flashlight test consistent with an afferent pupillary defect.
Patient suspected of having temporal arteritis should receive high-dose corticosteroid therapy immediately, treatment should NOT be delayed to obtain or confirm the biopsy results. Patients with visual loss should be admitted and given IV steroids.
Low dose aspirin is used to reduce the risk of permanent vision loss or stroke from vessel occlusion.
CVC
A central venous catheter (CVC) is commonly used for the administration of critical care medications (eg, pressors, hypertonic saline) and in the setting of difficult vascular access or need for long-term medication (eg, chemotherapy). The preferred points of central venous access are the internal jugular vein (typically by ultrasound guidance) or the subclavian vein (typically by anatomic landmark guidance). CVC complications occur in up to 15% of cases, and many are due to inappropriate catheter placement.
The ideal placement of a CVC tip is in the lower superior vena cava. Tip placement in smaller veins (eg, subclavian, jugular, azygous) predisposes to venous perforation. In addition, inappropriately placed catheter tips may cause lung puncture, leading to pneumothorax, or myocardial perforation, leading to pericardial tamponade. Arterial (eg, subclavian) puncture is also a risk, particularly in the absence of ultrasound guidance.
Confirmatory chest x-ray may be omitted in the setting of an uncomplicated (eg, first needle insertion, no resistance to catheter advancement) ultrasound-guided CVC placement. Otherwise, a portable chest x-ray should be performed immediately following CVC placement to facilitate timely recognition of a misplaced catheter tip, identify possible injury (eg, pneumothorax), and prevent exacerbation of injury (eg, infusion of fluid into the pericardial space). Visualization of the catheter tip just proximal to the angle between the trachea and right mainstem bronchus confirms appropriate placement.
Anterior Mediastinal Mass
The differential diagnosis for an anterior mediastinal mass includes the "4 T's":
thymoma
teratoma
thyroid neoplasm
terrible lymphoma
Within the category of teratoma, one must also include other germ cell tumors. Teratomas can often be distinguished from other germ cell tumors on imaging by the presence of fat or calcium, particularly if in the form of a tooth. Serum hormone levels may be helpful in differentiating seminomatous germ cell tumors from nonseminomatous variants. Serum β-HCG can be elevated in 1/3 of patients with a seminoma, although the AFP is essentially always normal. Nonseminomatous forms of germ cell tumors include yolk sac tumor, choriocarcinoma, and embryonal carcinoma. A mixture of different cell types is also possible and is referred to as a mixed germ cell tumor. Most patients with a nonseminomatous germ cell tumor have an elevated AFP, with a considerable amount also having an elevated β-HCG.
While seminomas often cause an elevated β-HCG, the AFP is essentially always normal.
MUGA
Multi Gated Acquisition Scan (MUGA) scan. The MUGA scan is the most accurate test in measuring ejection fraction. It is a noninvasive nuclear test that uses a radioactive isotope called technetium to evaluate the function of the ventricles (for example, before trastuzumab). Though not as accurate, an echocardiogram (B) is used more commonly because it is cheaper and more readily available and can look for valve function as well as focal areas of wall motion abnormality. Electrocardiogram (C) and exercise stress test are unable to measure a patient’s ejection fraction. Coronary angiography (D) is considered the gold standard in identifying coronary artery disease and can estimate ejection fraction, but is not as accurate.
Last updated