08 Infectious Disease
Bacterial
Osteomyelitis
Osteomyelitis is an infection localized to bone.
Osteomyelitis can develop through hematogenous spread, by local extension, or by direct inoculation.
Osteomyelitis in children typically follows bacteremia.
Bugs
Staphylococcus aureus is the most common overall cause of osteomyelitis.
Salmonella paratyphoid is a common cause of osteomyelitis in patients with sickle cell disease.
Pasteurella multocida is a classic cause of osteomyelitis in patients with a history of cat or dog bites.
Patients with a history of drug abuse often develop osteomyelitis caused by:
Candida
Pseudomonas
S. aureus
Risk factors for the development of osteomyelitis include:
Immunodeficiency
IV drug use
Diabetes mellitus
Recent trauma or surgery
Symptoms
The clinical presentation of osteomyelitis is nonspecific, with symptoms including:
Bone pain
Fever
Chills
Fatigue
Physical exam findings of osteomyelitis are tenderness over the affected bone and erythema.
Diagnosis
Lab findings in osteomyelitis show increased:
WBCs
ESR (usually >100)
CRP
A bone biopsy is necessary for cultures in order to determine the type of antibiotic treatment for osteomyelitis.
MRI is the most sensitive imaging modality for the diagnosis of osteomyelitis.
X-rays are relatively insensitive in diagnosing acute osteomyelitis (<10 days) but can be informative in diagnosing chronic osteomyelitis.
Treatment
Complications of osteomyelitis include amputation in improperly treated cases and pathologic fracture in acute pediatric cases.
Treatment of osteomyelitis involves antibiotic therapy for 4-6 weeks.
After a bone biopsy is obtained, initial antibiotic therapy for osteomyelitis involves broad-spectrum antibiotics until bone tissue culture results return.
Osteomyelitis frequently requires surgical debridement in addition to antibiotic therapy.
Syphilis
Treponema pallidum is the spirochete that causes syphilis.
Transmission of T. pallidum occurs via contact (sexual or casual) with skin lesions containing the spirochete, penetrating mucous membranes and causing systemic spread, or transplacentally.
Primary
Primary syphilis occurs after an average incubation period of two to three weeks and is characterized by a painless ulcerating papule known as a chancre. Chancres are highly infectious.
The chancre of primary syphilis heals in 14 days even without therapy.
The chancre from T. pallidum infection is noted to having a painless, non-exudative, clean, hard base with indurated margins ("punched out base with rolled edges"). This should be contrasted with granuloma inguinale from Klebsiella granulomatis infection, which presents with a painless ulcer with a beefy red base and irregular borders. In addition, this should be contrasted with a chancroid from Hemophilus ducreyi infection, described as a deep, undermined, painful purulent ulcer with soft ragged edges.
Secondary
Secondary syphilis occurs from a few weeks to months after primary syphilis and presents with a non-specific systemic illness, commonly referred to as the “great imitator.”
Maculopapular rash described as discrete copper, red or reddish-brown on the trunks and extremities, notably on the palms and soles.
Condyloma lata, which are raised, infectious, gray to white wart-like lesions found in moist areas and mucous membranes such as the mouth and perineum.
Systemic symptoms such as fever, headache, malaise, and myalgias.
Lymphadenopathy
Hepatitis
The word “condylomata” comes from the Greek word meaning “knob.” Two types of “knob-like” infections of the genitals are:
Condylomata acuminata (anogenital warts): dry skin-colored or pink papules caused by HPV
Condyloma lata (2° syphilis): moist papules covered by a gray exudate caused by T.pallidum*
In contrast to the latent phase of infection, patients with secondary syphilis are contagious.
Tertiary
Tertiary syphilis occurs years after untreated syphilis infection and can be divided into:
Gummatous syphilis
Cardiovascular syphilis
CNS syphilis
Gummatous syphilis is characterized by a gumma that can occur in the skin, bones, and internal organs. On the skin, gummas present as ulcers or granulomatous lesions with a round, irregular shape. Visceral gummas may present as a mass lesion. Gummas are usually absent of any causative organisms.
Cardiovascular syphilis classically affects the ascending thoracic aorta, manifesting as a dilated aorta and aortic valve regurgitation. The pathogenesis stems from a proliferative endarteritis that affects the vasa vasorum of the aorta, leading to medial necrosis and loss of elastic fibers.
CNS syphilis can be asymptomatic early on or may present with meningitis. Late neurosyphilis can include general paresis and/or more typically dorsal column demyelination, a condition referred to as tabes dorsalis. The constellation of findings in tabes dorsalis include:
Broad-based ataxia
Argyll Robertson pupil, sometimes referred to as a prostitute’s pupil, where the eye accommodates to near objects but does not react to light.
Positive Romberg sign, where swaying is noted when the person stands with eyes closed.
Charcot joints, a neuropathic arthropathy noted by the bony destruction and deformity of joints in this case due to decreased proprioception.
Stroke without hypertension
Congenital
Congenital syphilis occurs when T. pallidum is transmitted transplacentally from a pregnant woman to her fetus.
Hepatomegaly, with or without splenomegaly, and can also cause jaundice and cholestasis.
Rhinitis, also known as the “snuffles”, usually presents during the first week of life, and discharge contains spirochetes that can be transmitted by contact.
Maculopapular lesions on the palms and soles.
Generalized lymphadenopathy
Hematologic abnormalities such as anemia and thrombocytopenia
Frontal bossing and saddle nose
Interstitial keratitis
Sensorineural hearing loss
Hutchinson teeth and mulberry molars
Rhagades and gummas
Intellectual disability
Saber shins
Paroxysmal cold hemoglobinuria
ToRCHeS
Toxoplasmosis
Rubella
CMV
HIV
Herpes virus
Syphilis
Diagnosis
Diagnosis of syphilis is made via direct visualization using darkfield microscopy or direct fluorescent antibody testing, and via serology.
Serologic tests for syphilis include a screening test with a nontreponemal test such as the VDRL (venereal disease research lab) or RPR (rapid plasma reagin) test. A positive result is then confirmed as a true positive with a treponemal test, such as the FTA-ABS (fluorescent treponemal antibody-absorption) test.
Patients newly diagnosed with syphilis should also be tested for HIV.
False positive VDRL may be a result of:
Viruses (EBV and hepatitis)
Drugs (e.g. hydralazine and procainamide)
Rheumatic fever
Lupus and Leprosy
Treatment
Treatment depends on the stage of the infection.
The treatment for patients with primary, secondary, or early latent syphilis is a single dose of intramuscular penicillin G benzathine.
The treatment for patients with primary, secondary, or early latent syphilis that are allergic to penicillins is 2 weeks of doxycycline.
The treatment for patients with tertiary or late latent syphilis is intramuscular penicillin G benzathine given once per week for three weeks.
The treatment for patients with neurosyphilis is intravenous penicillin G benzathine given for two weeks. Patients with an allergy to penicillin should undergo desensitization.
Tuberculosis
Tuberculosis is caused by the bacteria Mycobacterium tuberculosis.
The incidence of tuberculosis has been slowly increasing in the past few decades because of the spread of HIV.
Tuberculosis most commonly occurs in the lungs, but it can spread to other sites, in which case it is called miliary tuberculosis.
Risk factors for tuberculosis include:
Immunosuppression (AIDS, malnourishment, immunosuppressants)
Alcoholism
Previous lung disease (e.g. COPD)
Diabetes mellitus
Advanced age
Homelessness
Tuberculosis can spread to other sites and cause meningitis and Pott disease, which is involvement of the bones of the spine.
Symptoms
Symptoms of tuberculosis include:
Cough
Hemoptysis
Dyspnea
Weight loss
Night sweats
Fever
Diagnosis
The first test used in the screening of patients for tuberculosis is the PPD (purified protein derivative) test. The size of the induration correlating with a positive result depends on certain factors.
5 mm - A PPD is considered positive at 5 mm if the patient is/has:
HIV positive
In close contact with a TB-infected patient
Signs of TB on CXR
Immunosuppresion
10 mm - A PPD is positive at 10 mm if the patient has a condition that increases the risk of reactivation, including:
Silicosis
Chronic renal failure requiring dialysis
Diabetes mellitus
Injection drug users
Health-care workers (including medical students!)
Incarcerated individuals
15 mm - A 15 mm induration is always considered positive.
Other tests include culture and acid fast stain of a sputum culture or sample obtained from a bronchoscopy.
CXR may show lower-lobe infiltrates in the case of a primary infection. Reactivation tends to occur in the upper lobes, so there will be apical fibronodular infiltrates and cavitation is often noted at apical locations.
Treatment
Tuberculosis is treated with a combination of drugs: isoniazid, rifampin, pyrazinamide, and ethambutol for 2 months followed by 4 months of just isoniazid and rifampin.
Vitamin B6 should be given with isoniazid since isoniazid causes a B6 deficiency.
Patients who are asymptomatic but have a positive PPD (a.k.a., latent tuberculosis in HIV-uninfected patients) should be treated with self-administered isoniazid (INH) for 9 months or INH and rifapentine for 3 months administered by direct observation.
Note that in some countries the BCG (bacillus Calmette-Guerin) vaccine is given, and these patients will always have a positive PPD. In such cases, a CXR or documentation of a recent CXR without symptoms is sufficient to rule out tuberculosis during routine screenings.
Note: Interferon-gamma release assays are also available as prior BCG vaccination does not produce a false-positive result.
Rheumatic Fever
Rheumatic fever is a systemic disease affecting the peri-arteriolar connective tissue of the heart. This can occur 1-4 weeks after an untreated group A streptococcal infection.
Autoantibodies can be made during a streptococcal infection which can attack joints and heart valves.
The heart valves most commonly affected by rheumatic fever in decreasing order of frequency are:
Mitral
Aortic
Tricuspid
Mnemonic: rheumatic fever: mitral > aortic > tricuspid
The incidence of rheumatic fever is low in the United States as a result of antibiotic treatment.
“Rheumatic heart disease” refers to acute carditis (pericarditis, myocarditis, valvulitis) and chronic valvular damage.
The incidence of rheumatic heart disease is only 3% of cases of untreated streptococcal infections.
Patients with rheumatic fever will typically present with:
Migratory arthritis, hot and swollen joints
Fever
Subcutaneous nodules on extensor surfaces
Sydenham’s chorea
Erythema marginatum
Diagnosis
The diagnosis of rheumatic fever is based on the evidence of a recent strep infection (e.g. serology such as ASO titer or anti-DNase B titer) and Jones criteria (discussed below).
Either ≥ 2 major Jones criteria or ≥ 1 major criterion and ≥ 2 minor Jones criteria are required.
Major JONES criteria include:
Joint findings, such as migratory polyarthritis
O, which represents carditis (think of the “O” as the rough shape of the whole heart, including all three layers-endocardium, myocardium, pericardium), which may include endocarditis, myocarditis, and/or pericarditis.
Nodules (subcutaneous) on extensor surfaces (eg, wrist, elbow, knee)
Erythema marginatum, which is a macular rash starting on trunk/arms
Sydenham’s chorea, which commonly affects the face and arms
Minor Jones criteria include:
Fever
Arthralgia (however, arthralgia cannot be used as a minor criteria if polyarthritis is counted as a major criteria)
Increased serum levels of acute phase reactants (eg, CRP, ESR)
Prolonged PR interval on EKG
The 5 “A findings” associated with rheumatic fever include:
A group β-hemolytic streptococcal pharyngeal infection
Aschoff bodies, which are sites of focal interstitial myocardial inflammation with a granuloma
Anitschkow cells, which are activated histiocytes with a rod shaped “caterpillar” nucleus
ASO titer elevated (anti-streptolysin O)
Antibodies to M protein (immune mediated type II hypersensitivity)
Treatment
Acute rheumatic fever should be treated with NSAIDs for joint inflammation and penicillin or erythromycin to eradicate Group A strep sources (even if pharyngitis is already resolved).
If a patient has severe carditis, corticosteroids may be necessary.
Surgery (such as mitral valve repair) might be required for valvular pathology.
Patients should be treated with penicillin or erythromycin in cases of strep pharyngitis to prevent progression to acute rheumatic fever.
Recommended prophylaxis with penicillin or erythromycin is indicated for:
Gastrointestinal, dental, and genitourinary procedures
prevention of recurrence in documented rheumatic fever
prevention of recurrence in documented rheumatic heart disease
Rocky Mountain Fever
Rocky mountain spotted fever (RMSF) is caused by the gram-negative, obligate intracellular bacterium _Rickettsia rickettsii. _It is most often transmitted by tick bites.
Rocky mountain spotted fever is most prevalent in southeastern and south central states NOT in the geographical distribution of the rocky mountains.
Symptoms
Patients with RMSF present with early nonspecific symptoms 2-14 days after being bitten such as:
Acute onset fever
Headache
Myalgias/Arthralgias
Nausea
A macular/petechial rash starts on ankles and wrists within 72 hours of disease presentation, then moves centrally to the trunk and eventually the palms and soles. The finding of the rash on the palms and soles is highly characteristic of RMSF but does not occur till the later stages of the disease.
Note the infections that cause palm and sole rashes can be remembered with mnemonic: You drive CARS with your palms and soles
Coxsackie A
Rocky Mountain spotted fever
Syphilis (secondary)
Diagnosis of RMSF is based on clinical examination in patients with consistent symptoms from an endemic area. There are currently no reliable diagnostic tests during the early phase of the illness when treatment should be initiated.
Thrombocytopenia is a useful diagnostic clue that can aid in the diagnosis of RMSF but may not be present in all patients with the infection.
Doxycycline is the drug of choice for adults and children with RMSF.
Chloramphenicol is the treatment of choice for pregnant woman diagnosed with RMSF.
Salmonella
Salmonella are gram-negative motile bacilli that are potential enteric pathogens and a common cause of gastroenteritis, with S. enteritidis being the most common cause.
Transmission of Salmonella most commonly occurs with consumption of contaminated food, such as eggs, poultry, beef, and vegetables.
Transmission can also occur with handling of certain animal species (e.g. turtles, snakes, kittens, and chickens).
Most patients infected with Salmonella present with
Nausea
Vomiting
Fever
Abdominal cramping
Watery diarrhea that typically resolves within 4 – 10 days
Salmonella is typically diagnosed based upon history (i.e. consumption of high risk food source) and presentation, but the most accurate diagnostic modality is a positive stool culture.
Salmonella gastroenteritis may result in complications such as:
Dysentery
Bacteremia
Endocarditis
Osteomyelitis
Most infections are self-limited and typically treated with fluid and electrolyte replacement.
In severe infections (e.g. high fever, persistent diarrhea, and bacteremia) patients may require antibiotic therapy. Fluoroquinolones (e.g. ciprofloxacin, levofloxacin) are first-line agents. Alternative therapies include trimethoprim-sulfamethoxazole, cefixime, or azithromycin.
Salmonella Typhi
Salmonella typhi (S. typhi) is a serotype of Salmonella enterica that causes typhoid fever, a potentially fatal multi-systemic illness characterized by fever, rash, abdominal pain and constipation.
S. typhi adheres to the lymph tissue (Peyer’s patches) within the terminal ileum where they are taken up by macrophages and enter the lymphatic system. The bacterium use macrophages to spread and infect other areas of the body.
Risk factors for the development of infection include traveling to endemic areas (South-Central Asia, Bangledesh, India, and Pakistan), consumption of contaminated food from food vendors, poor hand hygiene, and close contact with someone who is infected with the bacteria.
Symptoms
Clinical features of typhoid fever typically manifest within 5 – 21 days after inoculation.
During the 1st week of illness patients typically present with:
Rising (“stepwise”) fever (peaks at 103-104 degrees F)
Chills
Myalgias
Over the 2nd week of illness patients develop truncal rose spots (i.e. salmon-colored maculopapular patches) and diffuse abdominal pain.
During the 3rd week of illness patients may present with:
Severe abdominal distension
Hepatosplenomegaly
Sepsis
Weight loss
Diarrhea
Confusion
Psychosis
Culture isolation of the organism from either the bone marrow (most sensitive) or the serum is the diagnostic method of choice for Salmonella typhi. Imaging modalities such as radiography and CT scan are helpful in the diagnosis of bowel perforation, which can be a complication of the infection.
Typhoid fever may ultimately result in complications such as:
Intestinal perforation
Peritonitis
Kidney failure
Delirium
Psychosis
Death
Treatment of typhoid fever is typically 7-10 days of antibiotic therapy. Drugs of choice in adults are fluoroquinolones (ciprofloxacin or ofloxacin). In fluoroquinolone resistant regions, such as Asia, azithromycin can be used.
First-line treatment in children is cefixime.
Shigella
Shigella organisms are a group of non-motile, gram-negative pathogens responsible for causing shigellosis that are grouped into 4 species:
S. sonnei (most common in U.S.)
S. flexneri (most common in developing countries)
S. dysenteriae
S. boydii
Shigella bacteria invade the intestinal lining through the M-cells interspersed within the epithelial tissue. They then begin to multiply and cause an acute inflammatory reaction, which results in epithelial death and sloughing within the intestinal walls.
The primary mode of transmission of Shigella is via the fecal-oral route.
In the U.S. shigellosis is most commonly acquired in day care centers and crowded living conditions, such as urban centers.
Other risk factors include food borne transmission (cold potatoes, macaroni salads, and raw vegetables), and men who have sex with men.
In the developing world the most common mode of transmission is poor sewage disposal.
Symptoms
Most patients with shigellosis present with:
High fever
Abdominal cramping
Tenesmus (feeling of needing to pass stools despite an empty colon)
Bloody mucoid diarrhea
Diagnosis
The best diagnostic modality for Shigella is a stool culture.
Other helpful tests include fecal leukocytes and erythrocytes, and stool microscopy (isolates organism within the stool).
Shigellosis may result in complications, such as:
Intestinal or colonic perforation
Bacteremia
Tenesmus
Toxic megacolon
Metabolic disturbances
Hemolytic uremic syndrome (HUS)
Most Shigella infections are self-limited and typically only require supportive therapy with oral hydration.
If an individual has prolonged diarrhea or a worsening condition, antibiotic therapy can be initiated. The antibiotic of choice is a fluoroquinolone such as levofloxacin, which achieves high effective concentrations in the stool. Azithromycin or trimethoprim-sulfamethoxazole are alternatives. In patients with a high likelihood of drug-resistance, a third generation cephalosporin such as ceftriaxone is the empiric treatment. Sucsceptibility testing may be beneficial.
Tetanus
Tetanospasmin (tetanus exotoxin) is a protease that cleaves the releasing protein synaptobrevin, thereby inhibiting the release of inhibitory neurotransmitters glycine and GABA. Tetanospasmin therefore inhibits the inhibitor and causes net stimulation.
By inhibiting the release of glycine and GABA, tetanospasmin causes excessive muscle contraction (tetanus) and these signs of rigid paralysis:
Trismus ("lockjaw") due to jaw muscle contraction
Risus sardonicus ("ironic smile of tetanus") due to facial muscle contraction
Opisthotonos (pronounced arching of back) due to contraction of back extensor muscles
Tetanus is a clinical diagnosis based on consistent symptoms and history of inadequate vaccination. Wound cultures are not reliable.
Patients with clinical signs of tetanus should be immunized w/ tetanus/diphtheria toxoid: infection does not lead to immunity. Give toxoid if:
Pt has had < 3 doses throughout lifetime
> 10 yr since last booster dose
Unknown immunization status
Benzodiazepines (e.g. diazepam) are used to alleviate muscle tetany.
Human tetanus immune globulin is used to neutralize unbound toxin
Toxic Shock
Presentation of toxic shock syndrome (TSS) includes:
Flu-like symptoms (high fevers, headache, myalgia)
Gastrointestinal symptoms (nausea, vomiting, and diarrhea)
Symptoms of shock (hypotension and warm skin due to peripheral vasodilation)
Inflamed mucous membranes
Diffuse macular, erythematous rash
Since there is no test for TSS and blood cultures are often negative, a high index of clinical suspicion is important for diagnosis.
Toxic shock syndrome (TSS) is an acute, febrile, exanthematous illness that involves multiple systems with potential complications, including:
Shock
Renal failure
Myocardial failure
Adult respiratory distress syndrome
TSS results from hematogenous dissemination of toxins from Staphylococcus or Streptococcus that act as superantigens, activating T cells and resulting in massive release of cytokines. The toxin, not the bacteria, is the cause of the syndrome.
Toxic shock syndrome is most often associated with tampon use during menstruation, but can also occur with
Severe burns
Severe wounds
Insect bites
Secondary to toxic epidermal necrolysis (TEN)
Treatment for toxic shock syndrome is three-fold:
Hemodynamic stabilization with aggressive fluid replacement and vasopressors if needed
Eradication of infection source by removal of tampon, debridement and washing of wound
Empiric intravenous antibiotics: vancomycin plus clindamycin (to suppress toxin synthesis)
V. Cholera
Vibrio cholerae (V. cholerae) is a highly motile, gram-negative bacterium that thrives in brackish aquatic waters and is known for causing global pandemics of severe diarrhea.
Only pathogenic strains O1 and O139 that possess cholera toxin (CT) and toxin co-regulated pilus (TCP) are able to cause cholera.
The TCP allows the bacterium to attach and colonize the small bowel. The CT is an AB subunit toxin that allows persistent activation of adenylate cyclase, which results in increased chloride secretion and decreased sodium absorption, producing massive fluid loss.
Cholera is endemic in around 50 countries, but it most commonly occurs in Asia and Africa. Risks for the development of cholera include consumption of contaminated food or water sources and even having O-type blood.
Symptoms typically begin within 24 – 48 hours after consumption and classically present with severe, profuse watery diarrhea (> 250mL/kg in 24 hours), vomiting, and crampy abdominal pain.
Diagnosis
Rapid tests have been developed to diagnose the presence of bacterial antigens within a stool sample, but the most accurate diagnostic test for Vibrio cholerae is a stool culture.
V. cholerae may result in the following complications:
Severe hypovolemia
Hypokalemia,
Hypo/hypernatremia
Renal failure
Acidosis
Seizures
Arrhythmias
Death
Rehydration with either oral or IV fluids is the first priority in treating patients with cholera.
It is important to measure a patient’s stool output so they are not overhydrated.
Antibiotics (e.g. doxycycline, tetracycline, and ciprofloxacin) are incorporated in moderate to severe cases.
Vibrio Para
Vibrio parahaemolyticus (V. parahaemolyticus) is a gram-negative bacterium that primarily inhabits marine environments and is a common contaminant of seafood. Consumption of raw or undercooked seafood typically results in a self-limited gastroenteritis.
It is most commonly associated with the consumption of raw or improperly cooked seafood, especially oysters. Other risks include wound exposure to estuarine waters and handling infected seafood.
Most patients present with:
Nausea
Vomiting
Abdominal cramping
Watery diarrhea
Diagnosis
V. parahaemolyticus is diagnosed by serum and stool cultures, clinical presentation, and recent history of seafood consumption.
V. parahaemolyticus infections may result in the following complications:
Severe diarrhea
Dehydration
Wound infections
Sepsis (i.e. especially in patients with severe liver disease)
Death
Treatment of the gastroenteritis primarily consists of fluid replacement. In more severe infections such as sepsis, antibiotic therapy (e.g. doxycycline) should be incorporated.
Wound infections typically respond well to local wound care and oral antibiotics (e.g. tetracycline). More severe wound infections will require surgical debridement.
Lyme Disease
Lyme disease is a spirochetal infection caused by Borellia burgdorferi, which is transmitted by the Ixodes tick. It has both local and disseminated manifestations.
Lyme disease is the most common tick-borne infection in the United States and the disease occurs most commonly in the northeast.
The clinical presentation of Lyme disease includes:
Bell palsy (especially bilateral Bell palsy)
Arthritis
Kardiac block
Erythema migrans (classic “bull’s eye” rash)
Mnemonic: BAKE a key lyme pie.
Stage 1 of Lyme disease is the early localized stage and is marked by:
Erythema migrans
Fever
Headache
Fatigue
Erythema migrans is the pathognomonic "target" or "bull’s-eye" rash of Lyme disease. It has the following characteristics:
Most common on the thigh, inguinal region, and axilla
Expands outwards
Warm and usually nontender
Stage 2 of Lyme disease can occur weeks to months after infection and includes the following sequelae:
Bell’s palsy
Aseptic meningitis
Sensory-motor neuropathies
Cardiac involvement (most commonly myocarditis and AV block)
Symptoms associated with stage 2 include:
Intermittent flu like symptoms
Headaches
Neck stiffness
Fever/chills
Musculoskeletal pain
Symptoms associated with lyme meningitis include:
Mild fever
Headache
Photophobia
Cranial nerve palsies
Absent Kernig and Brudzinski signs
Stage 3 of lyme disease occurs a few months to years later and includes the following sequelae:
Monoarthritis or oligoarthritis
Chronic synovitis
Subacute encephalopathy
Polyneuropathy
Diagnosis
For patients with erythema migrans who live or have recently traveled to an endemic area, diagnosis can be made on clinical presentation alone. Serology will be negative in patients with early localized Lyme disease.
Lyme disease is diagnosed with sequential ELISA (screening test) and if positive, confirmatory western blot.
Antibiotics used to treat early localized Lyme disease (erythema migrans) in nonpregnant adults and children ≥8 years of age include either:
Doxycycline (first-line)
Amoxicillin
Cefuroxime
Pregnant patients or children under the age of 8 with early localized Lyme disease should be treated with amoxicilin or cefuroxime.
Note: Doxycycline (which is the first-line treatment for early localized Lyme disease in other populations) is contraindicated in pregnancy and in children <8 years of age due to its propensity to discolor teeth and impair bone growth.
Patients with early disseminated Lyme disease and facial nerve palsy but no CNS infection can be treated with one of the same agents recommended for early localized disease:
Doxycycline (first-line)
Amoxicillin
Cefuroxime
Patients with early disseminated or late Lyme disease (e.g. patients with heart block or meningitis) are treated with intravenous antibiotics. Appropriate agents include one of the following:
Ceftriaxone (first-line)
Cefotaxime
Penicillin D
A single dose of doxycycline is recommended as prophylaxis for patients with an Ixodes tick that has been attached from 36 to 72 hours.
Ehrlichiosis
Ehrlichiosis is an acute febrile infection caused by the genera, Ehrlichia, which are obligate intracellular organisms.
Ehrlichia chaffeensis infects mononuculear cells causing human monocytotropic ehrlichiosis (HME).
Ehrlichia chaffeensis is endemic to the southeastern and mid-Atlantic states and cause ehrlichiosis mainly in the months of May through July.
Ehrlichiosis is transmitted via the Lone Star tick.
The Lone Star tick feeds on white tailed deer, a major reservoir for Ehrlichia chaffeensis.
Ehrlichiosis has a median incubation period of 8 days with subsequent clinical manifestations that include:
Fever
Headache
Myalgia
Malaise
Severe manifestations of ehrlichiosis include:
Toxic shock-like or septic-shock like syndrome
ARDS
CHF
Hepatitis
Meningoencephalitis
Hemorrhage
Diagnosis
Diagnosis is suggested by fever with a known tick exposure.
Lab values for ehrlichiosis can include:
Leukopenia
Thrombocytopenia
Hepatic aminotransferase elevation
PCR can be helpful for confirmation of acute ehrlichiosis.
Treatment
Treatment for ehrlichiosis is commonly doxycycline.
Avoid doxycycline and use amoxicillin in pregnant females and children under 8.
Gonococcal Tenosynovitis
Gonococcal tenosynovitis is a sexually transmitted condition causing inflammation of tendon and sheath.
Women are generally asymptomatic causing a higher incidence of developing disseminated gonococcal infection.
Symptoms
Signs and symptoms of gonococcal tenosynovitis include:
Tenosynovitis (inflammation of a tendon and the sheath surrounding it)
Migratory polyarthritis/septic arthritis
Skin rash on distal extremities
The presentation of gonococcal tenosynovitis is typically a young sexually active teenager/adult presenting with constitutional symptoms and monoarthritis/oligoarthritis often progressing to migratory arthritis in a few days.
Can also present as a finger stuck in the flexed position (i.e. trigger finger tenosynovitis) with extension of pain along the tendon with passive extension.
The most commonly involved areas of the body include:
Knee
Wrists
Ankle
Hands
Diagnosis and Treatment
Aspiration if a subcutaneous purulence develops and/or biopsy are required for diagnosis.
The treatment includes ceftriaxone. Always treat presumptively for chlamydial infection given high co-infection rate. Add doxycycline or a zithromycin.
Bacillus cereus
Bacillus cereus (B. cereus) is a spore-forming gram-positive bacterium known for causing acute food poisoning.
Two distinct enterotoxins are produced by the bacterium, the emetic toxin (heat stable) and diarrheal toxin (heat labile). Presentation will depend upon which toxin is involved.
Foods associated with the diarrheal-type include:
Meats
Vegetables
Sauces
Foods associated with the emetic-type include:
Rice (most common)
Potatoes
Pasta
The diarrheal syndrome is characterized by diffuse diarrhea and abdominal cramping that typically resolves within 24 hours.
The emetic syndrome is characterized by:
Nausea
Abdominal cramping
Vomiting
These symptoms typically occur within 6 hours after ingestion and resolve within 24 hours.
B. cereus is typically diagnosed solely on a patient’s history and presentation. B. cereus can be diagnosed by culture and PCR techniques, but these are not typically offered by most hospitals.
B. cereus may result in complications such as:
Dehydration
Bacteremia
Endocarditis
Food poisoning from B. cereus is typically self-limited and does not require antibiotic therapy.
Clostridium Difficile
Clostridium difficile colitis is an iatrogenic infection as a result of antibiotics (e.g. fluoroquinolones, ampicillin, clindamycin) altering intestinal flora allowing C. difficile to flourish. This is the most common cause of nosocomial diarrhea.
C. diff produces two toxins:
Exotoxin A (enterotoxin), which chemoattracts neutrophils which release cytokines, causing mucosal inflammation and GI fluid loss
Exotoxin B (cytotoxin), disrupts the cytoskeleton by depolymerizing actin filaments, resulting in GI mucosal cell death and pseudomembranous colitis
Toxin assay:
Cytotoxin assay, is the gold standard and is the most sensitive C. difficile test. High cost and long turnaround time limit its use.
Enzyme immunoassay, is preferred for detecting toxins A and B and is a widely used test. It is highly specific, but only moderately sensitive (negative tests must be re-checked with an alternate exam).
Real-time PCR for C. difficile gene toxin
Treatment of C. difficile colitis involves:
Witholding the offending medication (typically clindamycin)
Metronidazole
Vancomycin PO (not absorbed in the GI tract) if the condition does not improve
Surgical evaluation should be sought for patients with severe disease, which is defined as any one of the following criteria:
WBC counts ≥20,000 cells/microL
Plasma lactate between 2.2 and 4.9 mEq/L
Peritoneal signs
Severe ileus
Toxic megacolon
Viral
HSV
Herpes simplex virus (HSV), is a recurrent viral infection of the mucocutaneous surfaces. It is caused by either HSV-1 or HSV-2.
HSV-1 is generally transmitted via respiratory secretions and saliva. HSV-2 is generally sexually transmitted.
HSV-1 typically causes lesions above the waist and HSV-2 typically causes lesions below the waist.
The primary infection of herpes simplex virus typically presents with more severe symptoms and a flu-like illness.
Because of oral sex practices, HSV-2 is increasingly found above the waist.
Herpes labialis are oral lesions commonly known as “cold sores.”
HSV keratoconjunctivitis is the most common infectious cause of corneal blindness in US.
Herpes simplex also causes temporal lobe encephalitis, which is the most common cause of sporadic encephalitis in US.
Primary HSV infection in children most commonly presents as gingivostomatitis. This leads to fever, pharyngitis and painful vesicular lesions leading to difficulties in eating, drinking, and swallowing.
Reactivation: after primary infection, the virus lies dormant in ganglion neurons. The virus reactivates when the host is under stress or is immunocompromised.
Specific clinical manifestations of HSV-2 include shallow, erosive genital lesions approximately 2 to 7 millimeters in size with pain and pruritus.
In AIDS patients, can present as deep perianal or genital ulcerations/fissures
As one of the “ToRCHeS” infections, a complication of herpes is infection of the newborn during delivery because of vertical transmission during pregnancy.
Diagnosis
The diagnosis of HSV infection can be made via:
Culture
PCR (most sensitive)
Tzanck smear
If HSV encephalitis is suspected, PCR of a CSF sample should be performed for diagnosis.
HSV encephalitis is associated with temporal lobe lesions, which can be seen on brain imaging.
A Tzanck smear of a sample taken from open vesicles will show multinucleated giant cells with intranuclear inclusion bodies. Note that these findings are also present in patients with varicella-zoster virus (VZV); thus, the Tzanck smear cannot distinguish between HSV and VZV.
Treatment of herpes simplex virus is aimed at reducing symptoms because it cannot be cured and consists of antiviral drugs such as acyclovir, valacyclovir, famciclovir.
Metabolites of these nucleoside derivatives interfere with the synthesis of viral DNA; therefore, they do not eradicate HSV-1 or HSV-2 during latency as the virus is not replicating.
HIV
HIV is a viral illness transmitted through exposure to infected blood that results in chronic, progressive immunodeficiency. Extensive basic science coverage of HIV can be found in our step 1 topic, here.
The two categories of HIV infection are acute HIV infection and chronic HIV infection. Acute HIV infection is the period of rapid viral multiplication.
The stages of chronic HIV infection are the following:
Asymptomatic infection
Early symptomatic infection
AIDS, which is a CD4 count <200 cells/microL or an AIDS defining illness
Advanced HIV infection characterized by CD4 count <50 cells/microL
After approximately 6 months, the viral load has reached its set point, or maximum viral load.
Acute
The acute HIV infection can be asymptomatic in 10-60% of individuals, causing the infection to go unnoticed.
Constitutional symptoms of the acute HIV infection include:
Myalgias
Fatigue
Fever
One of the most distinct manifestations of the acute HIV infection is mucocutaneous ulceration, which can occur on oral mucosa, the anus, the penis, or the esophagus.
The acute HIV infection may also present with:
Generalized rash following the onset of fever
Nontender axillary, cervical, or occipital adenopathy
Nausea, diarrhea, and weight loss
Acute HIV infection causes a febrile illness that closely resembles mono (eg, malaise, generalized lymphadenopathy). However, with acute HIV, rash and diarrhea are common and tonsillar exudates are uncommon.
Chronic
Patients with chronic HIV infection typically remain asymptomatic during treatment. When symptomatic, findings include:
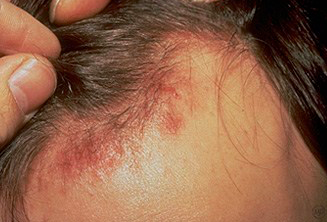
Persistent oropharyngeal or vulvovaginal candidiasis
Oral hairy leukoplakia
Seborrheic dermatitis
Opportunistic infections that patients with AIDS are predisposed to are covered in separate topics:
Opportunistic Infections: Cryptosporidium
Opportunistic Infections: CMV
Opportunistic Infections: Candidiasis
Opportunistic Infections: Toxoplasmosis
Opportunistic Infections: Pneumocystis Jirovecci
AIDS defining malignancies include:
Kaposi's sarcoma [KS] (HHV-8)
Cervical (women)/anal (men) cancer (HPV)
Non-Hodgkin lymphoma [NHL] including systemic NHL and CNS lymphoma (EBV)
Diagnosis
The diagnosis of suspected HIV infection is made with two tests. An ELISA or enzyme immunoassay is the screening test for HIV, which is then confirmed with a western blot.
An early HIV infection may produce a false negative result during HIV screening, but can produce a positive result with RT-PCR.
Mono
Infectious mononucleosis or heterophile-positive mononucleosis is a common viral infection in adolescents and young adults caused by the Epstein-Barr Virus (EBV).
Mononucleosis is most often transmitted by saliva, which is why it is often referred to as the “kissing disease.”
EBV first infects epithelial cells in the oropharynx/nasopharynx before spreading to B-cells in the blood and lymph nodes. Once infected, B-cells become antigenically stimulated, proliferate and produce nonspecific IgM antibodies which agglutinate upon exposure to pig, sheep, or horse red blood cells (heterophile antibodies).
Infectious mononucleosis is most common in adolescents and young adults ages 15-24. Most adults are considered carriers having experienced an asymptomatic EBV infection at a younger age. Previous infection generally confers lifelong immunity.
Patients with mononucleosis classically present with:
Malaise
Fever
Tonsillar pharyngitis
Generalized, painful lymphadenopathy (vs. lymphoma which is painless)
Hepatosplenomegaly
Even though the risk of splenic rupture is low, patients should still wait at least 3-4 weeks after the onset of symptoms before returning to strenuous activity or contact sports.
Patients with mononucleosis who are mistakenly treated for bacterial pharyngitis with ampicillin or amoxicillin (beta-lactams) are likely to present with a maculopapular rash.
The differential diagnosis for patients with symptoms of mononucleosis and a negative monospot test include:
HIV
Toxoplasmosis
CMV mononucleosis
Streptococcal infection
Lymphoma
EBV is associated with several forms of cancer including:
Burkitt’s lymphoma
Nasopharyngeal cancer
Hodgkin’s lymphoma - Mixed cellularity subtype
Primary CNS lymphoma
Posttransplant lymphoproliferative disorder
Complications from mononucleosis include:
Transient hepatitis
Splenic rupture
Thrombocytopenia
Hemolytic anemia
Upper airway obstruction due to lymphadenopathy
Guillain-Barre syndrome
Bell’s palsy
Increased long-term cancer risk from EBV infection
Diagnosis
All patients who present with symptoms of mononucleosis should be diagnosed with a Monospot (heterophile antibody) test and should have a WBC count with differential.
Additional laboratory findings consistent with mononucleosis include elevated liver enzymes (AST/ALT) and lymphocytic leukocytosis with large atypical CD8+ lymphocytes.
Patients with a negative or inconclusive monospot test and symptoms consistent with mononucleosis should have their monospot test repeated and/or EBV-specific antibody titers drawn.
The monospot test has a high false-negative rate in adolescents within 1 week of infection. It is also inaccurate when used to test infants and young children.
Treatment
The proper treatment for mononucleosis is supportive care. Patients should receive fluids for dehydration, adequate rest for fatigue, acetaminophen for fever and NSAIDs for throat pain.
A short course of high dose corticosteroids is recommended for patients with airway compromise and may also be beneficial in those who develop thrombocytopenia or hemolytic anemia. It is important that ONLY cases of complicated mononucleosis receive steroids to prevent adverse effects of immunosuppressive therapy.
VZV
Varicella (VZV) is an infection by the varicella-zoster virus (aka herpes zoster) that can present as both chickenpox (the primary disease) and shingles (which is the recurrent disease).
VZV infects and replicates in the respiratory tract (typically a 2 week incubation period) which progresses to viremia and then the development of an asynchronous vesicular rash. The virus establishes a lifelong latent infection in sensory ganglia (i.e., dorsal root ganglia).
The lesions associated with both chickenpox and shingles are small, red macules and papules with central vesicles (“dewdrops on a rose petal”) that become scabbed-over crusts. Note: rash lesions are asynchronous (i.e., are of different stages) as opposed to the synchronous rash lesions of smallpox, which are all in the same stage of development/evolution.
Complications for immunocompromised patients infected by VZV include the development of encephalopathy, retinitis, or VZV pneumonia.
Tzanck smear (no longer used but still commonly tested on exams) of samples taken from open vesicles may demonstrate multinucleated giant cells with intranuclear inclusion bodies in patients with HSV-1, HSV-2 or VZV.
Shingles represents the recurrent infection of varicella, which is more common in older patients who have already had a primary infection. VZV remains dormant in the dorsal sensory ganglia.
Unlike the wide distribution of lesions seen in chickenpox, shingles is limited to a single or few distinct dermatomes related to the ganglia in which the virus is dormant. Disseminated disease involves multiple dermatomes.
The lesions seen in shingles last for about a week and can be painful with tingling, shooting pain (compared to the pruritic lesions of chickenpox) and, as with chickenpox, are infectious until they crust over.
Treatment for shingles includes:
Analgesics
Acyclovir, especially for the immunocompromised, or those with lesions in the trigeminal nerve distribution as herpes zoster ophthalmicus is a sight-threatening condition related to VZV reaction in the trigeminal distribution.
Corticosteroids, only in conjunction with acyclovir for pain relief and improvement in healing.
Shingles vaccination is recommended for patients over age 60. It is contraindicated in highly immunocompromised patients.
Complications of shingles include postinfectious neuralgia, which is long lasting pain at the site of eruption, and trigeminal neuropathy.
ART
NRTI
Nucleotide and nucleoside reverse transcriptase inhibitors (NRTIs) are considered the backbone of the combination antiretroviral therapy for HIV. Additional antiretrovirals are added once drugs from this class have been selected.
These drugs are typically given in pairs, with the most effective currently being tenofovir (Viread) and emtricitabine (Emtriva). Tenofovir is nephrotoxic.
When tenofovir and emtricitabine are contraindicated, such as in renal failure with creatinine clearance <50 mL/min, abacavir-lamivudine (Epzicom) is the next best combination.
Protease Inhibitors
Protease inhibitors are an antiviral agent with a high barrier to resistance.
The preferred protease inhibitors to add to a NRTI backbone are atazanavir (Reyataz) or darunavir (Prezista) with the addition of ritonavir (Norvir) to either.
Darunavir should be avoided in patients with a sulfa allergy.
NNRTI
Non-nucleoside reverse transcriptase inhibitors (nNRTIs) are very potent, but are easily overcome by resistance.
Efavirenz (first generation), rilpivirine, and etravirine (both second generation) are the three drugs that are most often used from this class.
Efavirenz carries the risk of CNS toxicity.
Others
Maraviroc (Selzentry) is a CCR5 receptor antagonist and works by blocking entry of HIV into CD4 cells. It is not commonly used and is usually reserved for patients that have already been on treatment in which resistance is developing. It also requires a tropism assay to see if the patient will respond to a CCR5 antagonist.
Integrase inhibitors are a type of antiretroviral agent that work by preventing the incorporation of the viral genome into host DNA.
The integrase inhibitors include:
Dolutegravir (Tivicay)
Elvitegravir (Viteka)
Raltegravir (Isentress)
Dolutegravir is the preferred agent for the following reasons:
Lower chance of drug resistance
Can be used in renal insufficiency
Fewer drug-drug interactions
HIV Treatment
Treatment with antiretrovirals (ARTs) should begin immediately upon diagnosis, regardless of viral load or CD4 count. However, the patient must show willingness to be fully adherent to the ART regimen and attend follow-up appointments.
Highly active antiretroviral therapy (HAART) starts three drugs at the same time and should initially include:
Two nucleoside reverse transcriptase inhibitors
One protease inhibitor OR one non-nucleoside reverse transcriptase inhibitor OR integrase strand transfer inhibitor
Indications for changing HAART regimens include:
Failure to keep viral load under 50/mL
Poor compliance
Drug toxicity
Patients in virologic failure (viral load greater than 50/mL) should be tested for drug resistance, and have their HAART regimens adjusted.
HAART complexity can be decreased by including numerous drugs in one pill (combination pill).
Pregnant women should be treated in order to keep the viral load low and be given zidovudine during labor. This will lower the chance of vertical transmission.
Newborns should receive zidovudine for 6 weeks after birth and be checked for viral load. Anti-HIV antibodies will be present in HIV-negative neonates due to transmission from the HIV-positive mother.
Kaposi
Kaposi sarcoma is a condition caused by human herpes virus 8 (HHV-8) that results in angioproliferation.
When HHV-8 infects endothelial cells it causes increased expression of vascular endothelial growth factor (VEGF), resulting in angiogenesis.
There are four types of Kaposi sarcoma, which include::
Classic (Mediterranean or Ashkenazi Jewish)
Endemic (African)
Epidemic (AIDS-related)
Iatrogenic (transplant-related)
Symptoms
The clinical presentation of Kaposi sarcoma in an HIV patient is a rash that is non-responsive to multiple treatments.
The rash associated with Kaposi sarcoma is a purple-red-blue macules, nodules, or plaques as shown in the image.
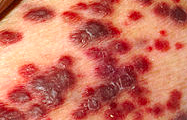
Cutaneous lesions can appear in the following locations:
Lower extremities
Face
Oral mucosa
Genitalia
Gastrointestinal symptoms can appear in AIDS patients including:
Abdominal pain
Nausea, vomiting, diarrhea
Upper or lower GI bleed
Intestinal obstruction
After observing characteristic lesions, including scoping for gastrointestinal lesions, the diagnosis of Kaposi sarcoma is confirmed with a biopsy of a lesion.
Currently there is no optimal treatment for Kaposi sarcoma, but the following can be considered:
HAART therapy for all HIV patients
Local chemotherapy for disfiguring lesions
Systemic chemotherapy for extensive disease/tumors with an aggressive course
Polio
Poliomyelitis is a viral infection that attacks the brain and anterior horn alpha motor neurons in the spinal cord. The disease has been nearly eradicated due to childhood vaccination.
Poliovirus is an RNA picornavirus, part of the enterovirus family.
More basic science information can be found in our Step 1 product here.
Polio is spread by the fecal-hand-oral transmission or by aerosol droplets.
The two vaccinations for the prevention of polio are the Salk (inactivated virus that is injected) and the Sabin (oral with attenuated virus).
The presentation of a poliovirus infection can vary and is generally divided into major and minor forms of illness.
The minor illnesses occur 1-3 days before paralysis develops and include:
GI complaints, such as nausea, vomiting, diarrhea, abdominal pain, and cramps
Sore throat
Headaches
Malaise
Fever
The major illnesses include all the CNS manifestations of the disease, such as:
Aseptic meningitis
Polio encephalitis
Bulbar polio
Paralytic poliomyelitis
Despite muscle weakness which may lead to paralysis, patients will have normal sensation (because only the alpha motor neurons are affected).
Serum findings associated with poliomyelitis include positive polio-specific antibody.
Lumbar puncture findings associated with poliomyelitis will be suggestive of viral meningitis, which are discussed here. A CSF viral culture can aid the diagnosis.
Treatment
Treatment of poliomyelitis involves supportive care and most patients fully recover.
Assisted respiration may be necessary if respiratory function is compromised as a result of paralysis.
Post-polio syndrome is a condition that may develop in polio survivors years after recovering from the initial infection. It typically causes progressive muscle weakness that can severely affect the ability to function independently.
Fungi
Candidiasis
Candidiasis is an opportunistic infection by the fungus Candida albicans.
Candidiasis typically causes infections in warm, moist areas, such as:
Oropharyngeal candidiasis
Vulvovaginitis
Esophagitis
Balanitis
Intertrigo
Oropharyngeal candidiasis (thrush) typically occurs in the following patients:
Infants
Elderly with dentures
Post-antibiotic
Immunocompromised
Patients using inhalant steroids for pulmonary conditions and local anti-inflammatory effects
Esophagitis presents as retrosternal pain upon swallowing. Candida esophagitis is considered an “AIDS-defining” illness.
Candidemia (may lead to infective endocarditis) can be seen in immunocompromised patients or patients in an ICU setting and presents with minimal symptoms such as low fever all the way to full blown sepsis.
Candida is a diploid yeast. Cultures show hyphae, pseudohyphae, or budding yeast cells. For most dimorphic fungi, the mnemonic "mold = cold, yeast = heat" applies, but Candida is the opposite. The mold form grows at 37C while the yeast form (pseudohyphae and budding yeasts) predominates at 20C. The formation of germ-tubes in culture (@ 37C) is diagnostic for Candida.
The treatment of Candida involves the use of azoles (e.g. fluconazole) and polyenes (nystatin, amphotericin B).
Azoles are teratogenic and should not be used in the first trimester.
Prophylaxis for candidiasis in immunocompromised patients is not recommended.
Coccidioidomycosis
Coccidioidomycosis is a granulomatous pulmonary infection caused by Coccidioides spp., which are soil-dwelling dimorphic fungi. It is also known as valley fever, San Joaquin fever, desert rheumatism, and coccidioidal granuloma.
Coccidioides spp. grow in the environment as spore-bearing mycelial forms. In their parasitic form, they appear as unique, endosporulating spherules in infected tissue.
Coccidioides spp. inhabit soil in arid regions.
C. immitis is primarily found in California’s San Joaquin Valley.
C. posadasii is endemic to southern regions of Arizona, Utah, Nevada, New Mexico, western Texas, and regions of Mexico and Central and South America.
Infection results from inhalation of spores. Incidence increases during windy, dry periods that follow rainy seasons. Seismic events, archaeological excavations, and other activities that disturb contaminated sites have caused outbreaks.
Inhaled spores reach terminal bronchioles where they transform into phagocytosis-resistant septated spherules in which many endospores develop. This leads to eventual granulomatous reaction.
Symptoms
Most cases of coccidioidomycosis are unrecognized. Vague respiratory symptoms may be reported. Early symptoms of coccidiodomyocis infection may include malaise, chills, fever, night sweats and chest discomfort.
The incubation period is 1-4 weeks, with an average of 10-16 days.
An evanescent, generalized, fine macular erythematous or urticarial eruption may be seen within the first few days of infection.
Erythema nodosum can occur (more often in women) and is sometimes accompanied by an erythema multiforme rash, usually 3-21 days after the onset of symptoms.
Hematogenous dissemination to the CNS can result in coccidioidal meningitis, a potentially fatal complication of infection that only occurs in 0.1% of infections.
In addition to meningitis, other extrapulmonary coccidioides infections include:
retropharyngeal abscess
prostatic infection
single cutaneous lesions
monoarticular arthritis
vertebral osteomyelitis
Diagnosis
Diagnosis is based on history of travel to an endemic area, plus one or more of the following:
Positive serology for anti-coccidioidal antibodies
Visualization of spherules on microscopy of a body tissue/fluid sample
Growth on body tissue/fluid cultures
The clinical constellation of erythema nodosum, fever, chest pain, and arthralgias (especially knees and ankles) has been termed "desert rheumatism" and "valley fever."
The chest examination is often normal. Dullness to percussion, friction rub, or fine rales may be present.
Chest radiograph may show unilateral infiltrate and/or ipsilateral hilar and mediastinal lymphadenopathy.
Treatment
Primary pulmonary coccidioidomycosis spontaneously resolves in 95% of patients without risk factors for dissemination; antifungal therapy does not lessen the frequency of dissemination. Patients should follow up at 3-6 month intervals for 2 years.
In an immunocompromised host or someone with severe symptoms, oral fluconazole or itraconazole or IV amphotericin B can be given.
PCP
Pneumocystis jirovecii (formerly P. carinii) is a fungal cause of pneumonia that is most commonly seen in HIV patients. It is known as pneumocystis pneumonia (PCP).
PCP is spread person to person by airborne contact. However, immunocompetent people have asymptomatic lung colonization.
Immunocompetence of both humoral and cellular responses are required to prevent PCP, both of which are compromised in HIV.
The typical presentation of PCP is seen in a HIV patient with CD4 <200 who experiences:
Non-productive cough
Fatigue
Chills
Fever
Chest x-ray of a patient with PCP classically shows diffuse, bilateral “ground glass” infiltrates extending from the perihilar region.
CT findings will show patchy or nodular ground glass attenuation.
Pneumocystis pneumonia can cause hyponatremia due to the syndrome of inappropriate antidiuretic hormone secretion (SIADH).
To diagnose PCP, BAL (bronchoalveolar lavage) may be silver stained which will show the presence of Pneumocystis jirovecii.
Histologically, pneumocystis pneumonia shows a foamy, eosinophilic alveolar exudate.
The first-line treatment for PCP is oral trimethoprim-sulfamethoxazole (TMP-SMX).
For patients with severe disease (pO2 <60 mmHg or A-a gradient > 35 mmHg), intravenous administration should be used.
Adjunctive corticosteroids (e.g. prednisone) in patients with severe Pneumocystis pneumonia (i.e. pulse oximetry <92%, PaO2 <70 mmHg on room air, or A-a oxygen gradient ≥35 mmHg) decrease the incidence of respiratory failure and mortality.
Corticosteroids should be started concurrently with the initiation of TMP-SMX in these patients since the lysis of Pneumocystis fungi can induce pulmonary inflammation and concomitant clinical deterioration within 2-3 days of starting antimicrobial therapy.
Prophylactic TMP-SMX should be initiated for all patients with a CD4 count <200 cells/microL. Transplant recipients and patients on high doses of immunosuppressive agents are also candidates for PCP prophylaxis.
Alternatives if sulfonamides cannot be tolerated include dapsone or aerosolized pentamidine.
Parasites
Schistomiasis
Schistosomiasis is caused by a genus of blood flukes called Schistosoma.
Initial signs and symptoms of schistosomiasis are dermatitis, which includes:
Localized erythema
Pruritic maculopapular rash
Acute
Acute schistosomiasis (Katayama syndrome) develops within 2-8 weeks and includes:
Acute onset of fever
Myalgias, Malaise
Abdominal pain
Hepatosplenomegaly
Headache
Urticaria
Cough
Diarrhea, potentially bloody
Lymphadenopathy
Chronic
Chronic schistosomiasis can result in scarring of of the bowel and bladder venules.
Chronic intestinal schisotomiasis can result in:
Portal Hypertension
Esophageal varices
Hepatic failure
Pulmonary hypertension and right ventricular failure
Chronic urinary schistosomiasis can result in:
Hematuria and dysuria
Hydroureter
Hydronephrosis
Bacterial urinary tract infections
Kidney or bladder cancer
Laboratory results for schistosomiasis can include:
Stool and urine examination for eggs
Leukocytosis and eosinophilia if acute infection
Treatment of schistosomiasis is praziquantel.
Lifecycle
Schistosomiasis is primarily due to an immune response to the eggs, which includes inflammation, granuloma formation, and fibrosis.
Four species of Schistosoma cause intestinal schistosomiasis through infection of mesenteric venules.
These species include:
Schistosoma mansoni that are endemic to Africa, the Arabian peninsula, South America, and the Caribbean
Schistosoma japonicum that are endemic in China and Southeast Asia
Schistosoma mekongi that are endemic by the Mekong river in Southeast Asia
Schistosoma intercalatum that are endemic in Africa.
Schistosomiasis occurs in freshwater via snails that carry miracidia, which are released in water as cercaria. After invading host tissue, the cercariae transform into schistosomula.
Schistosomula cause infection by:
Penetrating skin or mucous membranes
Migrating to the portal circulation
Maturing in 6 weeks
Mating
Migrating to the terminal mesenteric or bladder venules where females lay their eggs
Schistosoma eggs can subsequently:
Pass with urine or feces
Be retained in the bowel or bladder wall
Enter the circulation and be transported to other tissues, mainly the liver
Schistosoma haematobium, endemic to Africa and the Middle East, causes urinary schistosomiasis by infection of venules in the urinary tract.
Entamoeba Histolytica
Entamoeba histolytica (E. histolytica) is a non-flagellated protozoal parasite that is a well-known cause of amebic dysentery. The parasite is transmitted after a person ingests the cystic form. Once inside the small bowel, parasitic trophozoites are released and invade the colonic mucosa, leading to severe diarrhea.
E. histolytica is primarily transmitted via the fecal-oral route and infection can occur after consuming fecally contaminated water or food.
The majority of patients present with:
Abdominal pain
Weight loss
Diarrhea
Bloody stools
Mild fever
E. histolytica infections may result in complications such as:
Toxic megacolon
Intestinal perforation
Liver abscess
Fulminant colitis
Pericarditis
All infections require antibiotic treatment with metronidazole, even if a person is asymptomatic.
The best initial diagnostic test for E. histolytica is to search for cyst and trophozoites within a stool sample.
The most accurate diagnostic test for_E. histolytica_is an enzyme immunoassay (EIA) test, which detects serum antibodies specific for the organism.
Other helpful diagnostic tests include colonoscopy with biopsy, abdominal ultrasound, and abdominal CT exam. Imaging is helpful when extraintestinal manifestations are suspected, such as a liver abscess.
Giardia
Giardia intestinalis is a flagellated protozoan that has 2 morphological forms: trophozoites and cysts. Cysts are the infectious form of the parasite, which are a common cause of diarrhea and malabsorption after the consumption of contaminated water sources.
Following ingestion of the cyst, excystation occurs within the small bowel, releasing the trophozoites. Trophozoites subsequently attach themselves to the intestinal epithelium where they cause increased epithelium permeability and block absorption.
Trophozoites that do not attach to the small bowel travel towards the large intestine where they transform back into their cyst (infectious) form. The cysts are subsequently excreted with the stool.
Giardiasis most commonly occurs in hikers/campers that consume contaminated water sources (e.g. mountain streams), but can also occur with contaminated food. Day-care centers are another well-known source due to poor hand hygiene and increased contact with feces.
Symptoms typically develop after an incubation period of 7-14 days and may last between 2-4 weeks. Most patients present with:
Watery diarrhea
Foul-smelling and greasy stools
Flatulence
Weight loss
Malaise
The best initial diagnostic modality is an ova and parasite test, which uses a stool sample to look for cysts and trophozoites under microscopy.
The most accurate diagnostic modality is a direct immunofluorescent assay (DFA), which detects antibodies against the cysts or trophozoite antigens within the stool.
Giardiasis may result in the following complications:
Malabsorption
Weight loss
Growth retardation
Chronic giardiasis
Rash
Reactive arthritis
Treatment of symptomatic giardiasis typically includes:
Fluid and electrolyte replacement
Avoidance of lactose-containing foods for 1 month
Antibiotic therapy
The first-line medications for giardiasis include:
Metronidazole
Tinidazole
Nitazoxanide
Babesiosis
Babesiosis is an infection caused by the protozoa Babesia. The most common species that infects humans in the United States is Babesia microti.
B. microti is endemic in the Northeast and Islands such as Nantucket, Martha's Vineyard, eastern Long Island.
Humans are not the primary reservoir for Babesia,but are incidentally infected when bitten by the deer tickIxodes scapularis (which also transmits Lyme disease).
Babesia sporozoites invadered blood cells, ultimately leading to lysis of host red blood cells.
The clinical presentation of babesiosis can vary, but symptoms typically develop 1-6 weeks after a tick bite.
Mild symptoms associated with babesiosis include:
Fever
Chills
Sweats
Myalgia
Anorexia
Arthralgia
Severe symptoms associated with babesiosis include:
Anemia
Jaundice
Hemoglobinuria (secondary to brisk hemolysis)
Eventually, multi-organ system failure
Risk factors for developing severe disease include:
Male
Age > 50
Immunosuppression
Malignancy
Splenectomized patients (cannot clear RBCs)
Complications associated with babesiosis include:
Acute respiratory distress syndrome
DIC
Congestive heart failure
Renal failure
Jaundice and severe transaminitis
The definitive diagnosis of babesiosis made with a blood smear (using Wright or Giemsa staining), which will demonstrate round or pear shaped organisms in tetrads forming a “Maltese cross” pattern inside red blood cells.
Polymerase chain reaction (PCR) is more sensitive and can detect low level parasitemia.
Babesiosis is treated with antimicrobial therapy in symptomatic patients and in asymptomatic patients with parasites on blood smear or DNA on PCR for greater than three months:
Mild illnesses (10-day course)
oral Atovaquone + azithromycin
oral Clindamycin + quinine(more severe side effects)
Severe illness (hospitalized)
IV clindamycin + oral quinine
Persistent and relapsing (immunocompromised)
Atovaquone + doxycycline + azithromycin or clindamycin
Taenia Solium
Taeniasis is a parasitic infection caused by the tapeworm species Taenia solium (T. solium).
Human infection occurs after the consumption of undercooked pork meat infected with cysticerci (larva).
Cysticercosis develops by consuming the eggsthat are shed by a human carrier of the adult tapeworm.
Once ingested, the eggs hatch, penetrate the bowel and disseminate hematogenously to:
Striated muscle
Brain
Eye
T. solium is endemic toSouth and Central America, Africa, and Asia. Other risks include poor hand hygiene, and consuming raw or inadequately prepared pork.
Taeniasis most commonly presents with the following symptoms:
Crampy abdominal pain
Nausea
Diarrhea
Change in appetite
Cysticercosis presents primarily with muscular and neurological complaints:
Muscular pain and swelling
Seizures
Intracranial hypertension
Psychiatric disturbances
The best initial diagnostic test for taeniasis is to examine the stool for eggs and proglottids.
The most accurate diagnostic test is an enzyme-linked immunoelectrotransfer blot assay (EITB) of the serum.
Cysticercosis is diagnosed by imaging (CT or MRI) and enzyme-linked immunoelectrotransfer blot assay (EITB)of the serum or CSF.
Intestinal taeniasis is treated with fluid and electrolyte replacement and anthelmintic therapy; the drug of choice isalbendazole.
Treatment of patients with neurocysticercosis is primarily managed with antiepileptics (e.g. phenytoin, carbamazepine) andcorticosteroids, as anthelmintic therapy can exacerbate CNS symptoms.
T. solium may result in the following complications:
Severe dehydration
Nutrient deficiency
Seizures
Permanent brain damage
Psychiatric disturbances
Intracranial hypertension
Toxoplasmosis
Toxoplasmosis is a parasitic infection by Toxoplasma gondiithat is the most common CNS infection in untreated AIDS patients.
The disease is transmitted through the ingestion of infected meat or food contaminated with cat feces. Cats are the primary reservoir and definitive host.
Toxoplasmosis usually causes infection in AIDS patients when CD4 counts drop below 100 cell/microL.
Toxoplasmosis causesself-limited, flu-like disease in immunocompetent persons.
In immunocompromised, toxoplasmosis causes several manifestations throughout the body, including:
Pneumonitis
Myocarditis
Necrotizing encephalitis (multiple ring-enhancing lesions on CT)
Such ring-enhancing brain lesions can also be seen in AIDS lymphoma and abscesses. In addition, metastases are usually ring-enhancing but can sometimes cause uniformly enhancing brain lesions.
If a pregnant woman without previous exposure becomes infected, T. gondii protozoa may cross the placenta causing congenital toxoplasmosis.
Children may be born asymptomatic, but the classic triad of symptoms associated with congenital toxoplasmosis include:
Chorioretinitis (cotton-like white/yellow scars on the retina)
Hydrocephalus
Intracranial calcifications (ring-enhancing lesions in the cortex and basal ganglia on head CT)
Toxoplasmosis can be diagnosed with serology showing IgG antibodies positive forT. Gondii. Less commonly, brain tissue can be biopsied to show the presence of organisms.
Because toxoplasma gondii also causes calcified cysts in the brain, MRI (more sensitive than CT) can be used to detect the presence of CNS infection (“ring-enhancing lesions”). However, neither CT nor MRI can distinguish toxoplasmosis from similar CNS lesions.
The treatment of toxoplasmosis is pyrimethamine(most effective)and sulfadiazine.
Leucovorin (folinic acid rescue) should be given with pyrimethamine to avoid marrow suppression.
If intracranial pressure elevates during treatment, glucocorticoids should be administered.
Prophylaxis against toxoplasmosis should be initiated with TMP-SMX whenserologically positive for toxoplasmosis at the time of HIV diagnosis, CD4 levels drop below 100 if asymptomatic, or below 200 if an opportunistic infection or malignancy develops.
Cryptosporidium
The clinical presentation of Cryptosporidium in the immunocompromised host is chronic diarrhea with malabsorption and wasting if untreated.
Other manifestations of Cryptosporidium that have been found in AIDS patients include:
Cholecystitis
Cholangitis
Pancreatitis
Hepatitis
Respiratory tract involvement
In immunocompetent patients, infection causes self-limited diarrheal outbreaks that resolve in 10-14 days.
Nitazoxanide is the drug of choice for treating Cryptosporidium in immunocompetent children. In adults, the disease does not usually require treatment.
Treating Cryptosporidium in HIV patients requires the following:
Antiviral therapy to restore CD4 count
Supportive care
Nitazoxanide
There is no prophylaxis indicated in immunocompromised patients. Good hygiene and avoidance of high-risk water sources should prevent infection.
Cryptosporidium parvum (most common species) is a parasite that causes gastrointestinal disease and is one of the most common parasitic enteric pathogens in humans.
The target of infection and subsequent reproduction is epithelial cells of the digestive and respiratory tract.
Infection is mediated through the ingestion of fecally passedCryptosporidiumspores via contaminated water (most common), swimming pools, and animal contact.
Ingestion of only 10 oocysts can lead to severe disease in the immunocompromised host.
The diagnosis of Cryptosporidium can be made by identifying oocysts in the stool, but PCR is the diagnostic test of choice since it can identify specific genotypes. ELISA can be used, but is usually not due to high cost.
Ascaris Lumbricoides
Most infections are asymptomatic. When symptomatic, patients may show signs associated with the following categories:
Pulmonary and hypersensitivity reactions
Intestinal symptoms
Intestinal obstruction
Hepatobiliary and pancreatic symptoms
Pulmonary and hypersensitivity symptoms associated with A. lumbricoides include pneumonitis and urticaria.
Intestinal symptoms associated with A. lumbricoides include:
Anorexia
Nausea
Diarrhea
Malnutrition
Hepatobiliary and pancreatic symptoms associated with A. lumbricoides infection include:
Pancreatitis
Obstructive jaundice
Ascending cholangitis
Biliary colic
The first line treatment of A. lumbricoides involves the use of benzimidazoles (albendazole and mebendazole).
Second-line agents that may be used are ivermectin and nitazoxanide.
Ascaris lumbricoides is a helminthic infection that occurs most frequently in warm, wet, tropical climates.
Infection occurs by the fecal-oral route, typically by contaminated water or food.
The lifecycle of A. lumbricoides includes the following steps:
Ingested eggs hatch in the small intestine and mature into larvae.
Larvae invade and enter the bloodstream.
Larvae exit the bloodstream i n the lungs, penetrate alveoli, ascend up the respiratory tract and are swallowed.
In the small intestine, larvae mature into adult worms, which live in the lumen (do not attach to intestinal wall) and consume food, leading to malnutrition.
The female adult worms release eggs, which are passed out of the body via human feces.
The diagnosis of an A. lumbricoides infection can be made by detecting eggs in the stool (requires 40 days to appear once infected) and peripheral eosinophilia.
Malaria
Malaria is caused by different species of Plasmodium transmitted by the femaleAnopheles mosquito.
There are several species of Plasmodium that can cause malaria, each infecting red blood cells at different levels of maturity:
P. vivax/ovale only infect reticulocytes (immature RBCs)
P. malariae only infects mature RBCs
P. falciparum infects both reticulocytes and mature RBCs
P. falciparum causes the most severe form of malaria due to its ability to obstruct microcirculation leading to vascular occlusion, hemorrhage and necrosis. It is for this reason, P. falciparum can cause "cerebral malaria" (mental status changes / coma), renal failure and pulmonary edema.
Suspect malaria in a patient with a febrile illness and a history of recent travel to an endemic area (e.g. sub-Saharan Africa). Manifestations include:
Cyclical fever
Anemia
Thrombocytopenia
Hepatosplenomegaly
Nonspecific constitutional (e.g. chills, myalgias, headache) and gastrointestinal (e.g. nausea, vomiting, diarrhea) manifestations are also common.
Fever patterns depend on the species:
P. falciparum causes constant fever
P. ovale and P. vivax fever spikes every 48 hours
P. malariae fever spikes every 72 hours
P. vivax and P. ovale are the two species of Plasmodium that can form hypnozoites, a form of sporozoite that may remain dormant in hepatocytes and reactivate months/years after initial infection (relapsing malaria).
In order to eradicate hypnozoites, add a two week course of primaquine phosphateto any suspected infection of P. vivax and P. ovale.
The best test to diagnose malaria is a peripheral blood smear with Giemsa-staining.
The treatment of malaria is largely dependent upon where the patient acquired malaria. All areas of travel including sub-Saharan Africa, India, Oceania, Central America most of South America are chloroquine-resistant. Mexico and Argentina are the two areas of travel where it is safe to use primaquine for both prophylaxis and treatment.
Several drugs can be used for treatment of malaria in areas of chloroquine resistance:
Quinine sulfate + tetracycline
Atovaquone-proguanil
Artemether-lumefantrine
Chloroquine is the prophylactic drug of choice in areas still sensitive to it. Mefloquine is the prophylactic drug of choice for patients travelling to areas where chloroquine resistance has been reported.
Trichinella Spiralis
Trichinella spiralis is a nematode responsible for trichinellosis. In the contiguous United States, pigs are the most important reservoir. In Alaska, bears fill this role.
Humans are the end stage host of trichinellosis when encysted larvae in infected undercooked meat is ingested.
Once ingested, T. spiralis larvae invade and mature in the small bowel. Here, mature females release larvae into the blood where they encyst in striated muscle.
Characteristic locations of larvae invasion include:
Brain
Heart
Skeletal muscle
Diagnosis of trichinellosis is usually made clinically, but is confirmed with serology.
Less commonly, a muscle biopsy can be performed to demonstrate encysted larvae.
Eosinophilia can be seen on CBC.
Most cases of trichinellosis are self-limiting and can be treatedsymptomatically with analgesic and antipyretic agents.
Severe infections can be treated with benzimidazole drugs, which include:
Mebendazole
Albendazole
Thiabendazole
The most common presentation of trichinellosis is after encystation of skeletal muscle occurs, which presents with:
Muscle pain, tenderness, and swelling
Weakness
Periorbital edema
Cardiac involvement, while not common, can cause severe eosinophilic myocarditis.
Neurologic involvement, seen in up to ¼ of patients with trichinellosis, typically presents with a headache that is worse with movement.
Fever
For practical purposes, any single T > 38.2 °C or persistent T > 38.0 °C represents fever and should prompt further evaluation.
The most accurate methods for approximating core body temperature in the hospitalized patient include thermistor-equipped catheters placed in the pulmonary artery, esophagus, or urinary bladder (with bladder catheters being the most practical).
Oral temperatures tend to underestimate core body temperature by 0.5 °C.
Rectal temperatures tend to overestimate core body temperature by 0.3 °C.
The pathogenesis of fever involves release of inflammatory cytokines (released during inflammation) that act on the hypothalamus to elevate the body temperature.
When fever occurs secondary to infection, the severity of the feverdoes not correlatewith severity of the infection.
Initial considerations should include common nosocomial infections, including pneumonia, bloodstream infections, and urinary tract infections.
Surgical site infections are common in postoperative patients. Sternal wounds demonstrate a particular propensity to develop deep tissue infection; deep sternal wound infection is also known as mediastinitis and is a surgical emergency.
In patients with chronic indwelling nasogastric (NG) tubes, paranasal sinusitis is a common site of occult infection.
Given the widespread use of antibiotics, _Clostridium difficile_infection should always be suspected in a patient with fever and new-onset diarrhea.
In patients who have had abdominal surgery, abdominal abscess needs to be considered.
Endocarditisshould be a consideration in febrile patients with prosthetic heart valves and/or valvular abnormalities.
In patients who have had recent neurosurgical intervention, there should be a low threshold for ruling-out meningitis.
In cirrhotics and/or other patients with ascitic fluid, consider spontaneous bacterial peritonitis.
Fever is common in the post-operative setting due to tissue injury sustained by invasive procedures. Note that although atelectasis and fever often coincide post-operatively, atelectasis is not a cause of fever (which is a common misconception).
Venous thromboembolism can cause fevers, more commonly withpulmonary embolism than DVT.
Febrile non-hemolytic transfusion reactions occur sometimes with erythrocyte transfusions, and often with platelet transfusions.
A variety of drugs can be responsible for eliciting a “drug fever”. Particular attention should be paid to penicillins and cephalosporins due to their frequent use and common propensity to cause fever.
When fever cannot be readily explained by history and physical, at least three sets of blood cultures (ideally while patient is off antibiotics) should be obtained over a period of several hours; other fluids can be cultured based on suspicion.
Inflammatory markers such as ESR and CRP are helpful to rule-out presence of a significant inflammatory process.
Other screening tests include:
Infectious: HIV antibody and viral load, heterophile antibody test, tuberculin skin test and interferon-gamma release assay
Autoimmune: rheumatoid factor, antinuclear antibodies
Neoplastic: LDH, serum protein electrophoresis
Abdominaland chest x-ray are reasonable imaging studies to start with; tagged leukocyte and FDG-PETscans can be pursued when all other approaches have been exhausted.
A fever of unknown origin (FUO) is declared when:
The patient has had T >38.2°C on multiple occasions
The fever has lasted at least three weeks
At least one week of diagnostic work-up has been conducted in the hospital
Vancomycin (MRSA bloodstream coverage) and piperacillin/tazobactam (Gram-negative aerobic bacilli, including Pseudomonas coverage) is a common empiric regimen.
An antifungal agent can be considered in critically ill patients who have been on total parenteral nutrition with persistent fever on empiric antibiotics.
Antipyretics are not routinely indicated, except in patients where fever can lead to poor outcomes (e.g., increased intracranial pressure, stroke) or in patients with T > 41 °C, which is the "critical thermal maximum".
Neutropenic Fever
Neutropenia is defined as having an absolute neutrophil count (ANC) (neutrophil granulocytes) of less than 1500/mm3. ANC less than 500/mm3 carries a very high risk for infection.
Common causes for neutropenia are the following:
Bone marrow failure: drugs, toxins, antineoplastic agents
Bone marrow invasion: hematologic malignancy, metastatic cancer
Isolated neutropenia: agranulocytosis
Work up for neutropenia consists of the following:
CXR to look for signs of pulmonary infection
Pan-culture (blood, urine, sputum, LP)
CBC with differential to evaluate agranulocytosis
CMP should always be obtained
A fever in the setting of neutropenia is a medical emergency. The patient should be admitted to positive pressure room and strict contact precautions.
Treatment: Broad spectrum antibiotics, such as cefepime (cultures to be drawn prior to giving abx)
If fever persists after 5 days of broad spectrum abx
Start antifungals: IV Amphotericin B
Consider G-CSF
Agranulocytosis may be caused by the following drugs:
Ganciclovir
Clozapine
Carbamazepine
Colchicine
Methimazole
Propylthiouracil
Mnemonic: Gangs CCCrush Myeloblasts and Promyelocytes
Opportunistic
Infectious Agent
CD 4 Count
Prophylaxis
Pneumocystis jiroveci
<200
TMP-SMX
Toxoplasmosis
<100
TMP-SMX
MAC
<50
Azithromycin
CMV
Routine prophylaxis not recommended
Coccidiodes
<250 + endemic area
Itraconazole or Fluconazole
Histoplasma
<100 + endemic area
Itraconazole
Candida
Routine prophylaxis not recommended
At CD4 count <250: Fluconazole or Itraconazole for Coccidioidomycosis ONLY for patients in endemic areas.
At CD4 count <200: Add TMP-SMXfor Pneumocystis jirovecii(PCP)
At CD4 count <100: Toxoplasma is covered by TMP-SMX. Add itraconazole for Histoplasmosis prophylaxis ONLY for patients in endemic areas.
At CD4 count <50: Add azithromycin for Mycobacterium avium complex (MAC)
Last updated