Spinal Cord Syndromes
Peripheral Neuropathy
Peripheral neuropathy is any disorder of the peripheral nervous system including radiculopathies and mononeuropathies. For more information on specific peripheral neuropathies see: cervical radiculopathy, lumbosacral radiculopathy, and diabetic neuropathy.
Causes
There are many causes of peripheral neuropathy:
Diabetes mellitus
Alcohol abuse
B12 deficiency
B6 deficiency/ toxicity
Heavy metal exposure (eg. lead)
Rheumatologic
HIV
Spinal disk lesions
Common medications that cause peripheral neuropathy:
Dapsone
Daptomycin
HAART therapy
Chemotherapies such as vincristine and cisplatin
Isoniazid
Symptoms
Peripheral neuropathy typically presents with distal sensory loss, burning, or weakness.
Asymmetric muscle weakness can indicate:
Potential nerve infarction (diabetes)
Vasculitis
Entrapment
Trauma
Radiculopathy
Cerebral vascular accident
In the setting of a viral illness, most commonly with gastrointestinal symptoms, followed by acute weakness, consider Gullain-Barre syndrome.
Peripheral neuropathy of the hands and feet, angiokeratomas, cardiovascular and renal disease consider Fabry’s Disease.
Patients with a lead exposure will present with a peripheral neuropathy that frequently manifests with extensor weakness or "wrist/ankle drop" due to an axonal degeneration that primarily affects motor nerves.
Patients with chronic neuropathy associated with weakness may present with muscle wasting. This is typically seen in carpal tunnel syndrome.
If the etiology of the patient’s peripheral neuropathy is unclear, perform a nerve conduction study with EMG to differentiate between neuropathy and myopathy. EMG in neuropathy will reveal denervation potentials, fibrillations and positive sharp waves while EMGs studies in myopathies will reveal motor unit action potentials that are small and polyphasic.
Treatment
In general, the treatment for a peripheral neuropathy is to identify and treat the underlying cause.
Cervical Radiculopathy
Causes
Cervical radiculopathy, one of the most common causes of neck pain, refers to the damage of a cervical nerve root that transmits pain in a radicular fashion (e.g. follows a dermatome).
Cervical radiculopathy is most commonly caused by cervical spondylosis and disc herniation. Less common causes include:
Herpes zoster
Infarction
Nerve root avulsion
Mass effect from a tumor
Demyelinating disorders
Diabetic neuropathy
The timeline of the onset of cervical radiculopathy can help aid in diagnosis:
Acute onset typically results from disc herniation
Insidious onset typically results from cervical spondylosis
Cervical Spondylosis
Cervical spondylosis is the result of disk degeneration:
Aging disks fragment, lose water, and collapse. Initially starts in the nucleus pulposus:
Central annular lamellae buckling inward while the external concentric bands of the annulus fibrosis bulge outward. This causes increased mechanical stress at the cartilaginous end plates.

Subperiosteal bone formation occurs next, encroach on nervous tissues and cause irritations.
Ossification of the posterior longitudinal ligament, a condition often seen in certain Asian populations, can occur with cervical spondylosis. This condition can be an additional contributing source of severe anterior cord compression.
Disc Herniation

The most common site of cervical radiculopathy is at the level of the C7 nerve root.
Symptoms
Patients with cervical radiculopathy typically present with:
Acute or chronic onset
Neck, back, or arm pain that follows a dermatome
Paresthesia or numbness following a dermatome
Lhermitte's phenomenon (an electric shock down the arm following neck flexion)
Strength is usually preserved
Pseudo-anginal pain may imitate a myocardial infarction
Associated symptoms suggestive of another process (e.g. fever, weight loss, incontinence, gait disturbances)
On physical examination, patients with cervical radiculopathy typically have:
Diminished reflexes
Dermatomal changes in sensation and strength
Spurling maneuver, having the patient extend the neck, rotate the head towards the side of the pain and have the physician apply downward pressure. If pain occurs then the test is positive.
Midline neck tenderness (for disc herniation)
Diagnosis
Cervical radiculopathy is typically diagnosed through history and physical examination. Imaging and electrophysiologic (e.g. EMG) testing is indicated if symptoms do not resolve in 4 - 6 weeks or if there is localizing findings (e.g. weakness along one myotome).
For cervical radiculopathy, imaging studies are used to confirm the diagnosis and show displacement of the CSF or compression of the nerve root. The preferred imaging studies include:
MRI as the study of choice
CT myelography is the gold standard

Management
Cervical radiculopathy is initially managed with conservative therapy including:
NSAIDs
Short course of oral prednisone (Deltasone)
Physical therapy
Cervical traction
Cervical radiculopathy that is refractory to conservative therapy or causes severe symptoms (e.g. myelopathic findings) is managed with:
Epidural steroid injections (preferred over surgery)
Surgery(e.g. anterior cervical discectomy with fusion, disc replacement)
Lumbosacral Radiculopathy
Roots of all lumbosacral nerves originate in the T10 – L1 region, descend forming the cauda equina and then exit the spine at their respective levels
Cause
The etiology of lumbosacral radiculopathy includes spinal stenosis or disc herniation leading to compression of the nerve root.
Symptoms
Clinical presentation of lumbosacral radiculopathy depends on the level of the herniation or stenosis.
Radiculopathy at L1 is rare and presents with sensory loss and paresthesia in the inguinal area.
Nerves at level L2,3,4 innervate anterior thigh muscles and are responsible for hip flexion and knee extension. Radiculopathy at these levels are commonly seen in elderly patients with spinal stenosis and will present as an acute back pain with radiation to the anterior aspect of the thigh.
L5 is the most common location for a disc herniation. Herniation of the L4-L5 disc compresses the L5 nerve root (responsible for great toe dorsiflexion), which presents with back pain that radiates down the lateral (vs posterior in S1 compressions) aspect of the leg in to the foot.
S1 nerve root is responsible for ankle plantar flexion. S1 radiculopathy presents with back pain that radiates down the posterior aspect of the leg in to the foot.
Radiculopathy at levels S2, 3, 4 is rare and presents with sacral/buttock pain radiating into the perineum. It can also be associated with urinary or fecal incontinence and impotence.

Diagnosis
Urinary or fecal incontinence, new onset sexual dysfunction or saddle anesthesia are worrisome signs that need to be urgently addressed. The next step would be to obtain MRI and neurosurgical consult.
While the diagnosis of lumbosacral radiculopathy is most often clinical (based on sensory, motor and reflex deficits), neuroimaging with MRI (preferred)or CT scan is recommended for high-risk patients (e.g. progressive neurologic deficits, bladder dysfunction, saddle anesthesia, high suspicion for tumor or epidural abscess).

Treatment
Conservative treatment is the first step in management of lumbosacral radiculopathy. This includes:
NSAIDs
Prednisone can be used in severe pain
Physical therapy
Neurosurgical intervention is warranted if patient develops saddle anesthesia, urinary or fecal incontinence, sexual dysfunction.
Diabetic Neuropathy
Diabetic neuropathy is the progressive loss of nerve fiber function as a result of diabetes. Diabetic neuropathy is the most common complication of diabetes, affecting up to 50% of patients with diabetes.
Pathogenesis
As excess glucose is converted to sorbitol, the accumulation of sorbitol and fructose in neurons causes:
Reduced nerve myo-inositol, which is important in maintaining the cell membrane potential
Decreased membrane Na/K-ATPase activity
Impaired axonal transport
Structural breakdown of the nerve
Symptoms
Neural damage and conduction defects associated with diabetic neuropathy lead to sensory, motor, and autonomic symptoms.
Sensory symptoms typically begin in the feet and then progress in a stocking-glove pattern.
Sensory symptoms include:
Paresthesias
Neural pain
Decreased vibration and position sense
Loss of pain sensation
Motor symptoms may present distally or proximally and can be characterized by weakness or loss of coordination.
Motor symptoms may include:
Difficulty with fine hand motor coordination, such as turning keys or opening jars
Foot slapping, toe scruffing, and frequent tripping
Difficulty raising arms above the shoulders
Autonomic symptoms associated with diabetic neuropathy can include:
Postural hypotension
Impotence
Incontinence
Diabetic gastroparesis
The most common cranial nerve impacted by diabetic neuropathy is CN III. This neuropathy is caused by ischemia and infarction of CN III motor fibers that spares parasympathetic fibers. Thus, patients present with:
Diplopia
Ptosis
Fixed, "down and out" gaze
Preserved pupillary accommodation (spared parasympathetic fibers)
Diagnosis
The evaluation of diabetic neuropathy should include electrophysiologic testing of nerve function, including the use of nerve conduction velocity (NCV) or electromyography (EMG).
Management
The first line treatment for diabetic neuropathy is to control diabetes.
Neural pain can be treated with:
Tricyclic antidepressants
Phenytoin
Carbamazepine
Gabapentin
Persistent neural pain may require the use of narcotics or tramadol.
Preventative measures include teaching patients how to perform regular foot examinationsto avoid complications associated with diabetic neuropathy (explained below).
Complications
Complications associated with diabetic neuropathy include:
Charcot joints (also known as neurogenic arthropathy)
Diabetic ulcers, which can result in amputation from progressive infections and deformity
An increased risk of silent MI due to impaired pain sensation
Increased risks for epidural abscesses (unilateral UMN signs)
Conus Medullaris
In adults the spinal cord terminates at the level of L1-L2 in a tapered structure know as the conus medullaris. Sacral segments S1-S5 originate from the conus medullaris.
Causes
Causes of conus medullaris syndrome include spinal cord compression due to:
Trauma
Lumbar disc herniation
Tumors
Epidural abscess
Given that the conus medullaris is the last true segment of spinal cord and gives off the nerve roots S1-S5, conus medullaris syndrome generally manifests as a combination of BOTH lower motor neuron and upper motor neuron signs:
Upper motor signs include hyperreflexia, spasticity, Babinski sign.
Lower motor signs include hyporeflexia/areflexia, fasciculations.
One should suspect conus medullaris syndrome in a patient who presents with the following:
Sudden, symmetric bilateral leg weakness
Lower back pain
Hyperreflexia of lower extremity DTRs
Numbness to perianal area
Impotence
Management
Like cauda equina syndrome, conus medullaris syndrome is considered a medical emergency and requires urgent referral to neurosurgery for further management.
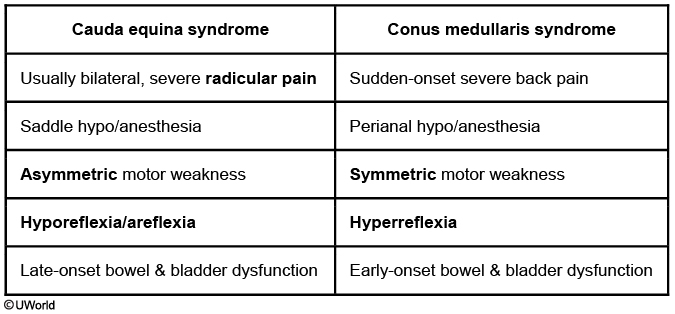
Conus medullaris syndrome can be confused with cauda equina syndrome. While both are considered medical emergencies, it is important to differentiate the two in clinical practice and for testing purposes.
Cauda Equina Syndrome
Conus Medullaris Syndrome
Onset and Pain
Gradual severe radicular pain
Sudden back pain
Symmetric vs. Assymetric
Assymetric
Symmetric
UMN vs. LMN
LMN
UMN + LMN
Nerve roots affected
L1-S5
S1-S5
DTR
Hyporeflexia/Areflexia
Hyperreflexia
Sexual dysfunction
Infrequent
Frequent
Bladder/Bowel incontinence
Late in disease course
Early in disease course
Cauda Equina
Cauda equina syndrome is an emergent condition that refers to the compression of the lumbosacral nerve roots (L1-L5, S1-S4) distal to the conus medullaris, resulting in neuromuscular and urogenital symptoms.
Causes
Cauda equina syndrome may occur secondarily to:
Trauma
Vertebral compression fractures
Disc herniation
Space occupying malignancies
Epidural abscess (by mass effect or vascular compromise)
Spinal stenosis (may be from osteoarthritis)
Inflammatory processes (e.g. ankylosing spondylitis, tuberculosis)
Cauda equina syndrome secondary to neoplastic compression is most commonly associated with:
Prostate cancer
Multiple myeloma
Lung cancer
Breast cancer
Symptoms
The classic triad of cauda equina syndrome is:
Saddle anesthesia (anesthesia in perineal region)
Bowel and/or bladder dysfunction
Lower extremity weakness
Diagnosis and Management
Urgent MRI of the spinal column is the preferred initial imaging modality to evaluate a patient with suspected cauda equina syndrome.
The management of cauda equina syndrome includes:
Emergent surgical decompression (e.g. laminectomy)
Immediate administration of dexamethasone (Decadron)
For malignancies, radiation or chemotherapy postoperatively
If left untreated, cauda equina syndrome may cause permanent nerve damage.
ALS
Pathogenesis
Amyotrophic lateral sclerosis (ALS) is a neurodegenerative disorder causing muscle atrophy and weakness, that ultimately leads to death.
The spinal areas affected in ALS are the corticospinal tract and ventral horn of the spinal cord. The disease affects both the upper and lower motor neurons but spares sensory neurons. It is characterized by neuronal death with gliosis replacing the lost neurons. The etiology of the disease is unknown.
Amyotrophic lateral sclerosis is classified as either sporadic or familial. The sporadic form is the most common, accounting for 90-95% of all cases of ALS.
Onset of the disease occurs between 50 and 70 years of age.
Amyotrophic lateral sclerosis has a poor prognosis with 80% mortality in 5 years.
Symptoms
Amyotrophic lateral sclerosis hallmark feature is the presence of both upper and lower motor neuron symptoms. Presenting symptoms include:
Progressive muscle weakness
Muscle spasticity
Fasciculations
Muscle atrophy
Speech and swallowing impairment
The following are never affected in amyotrophic lateral sclerosis:
Sensation
Extraocular muscles
Sexual function
End-stage amyotrophic lateral sclerosis is characterized by respiratory failure.
Diagnosis
Amyotrophic lateral sclerosis is primarily a clinical diagnosis based on the progressive symptoms of both upper and lower motor neuron degeneration.
Sensory and motor nerve conduction studies and electromyography are a standard part of the evaluation of motor neuron disease.
Neuroimaging can be done to rule out other possible diagnoses in a presumed amyotrophic lateral sclerosis patient.
Treatment
Treatment of amyotrophic lateral sclerosis is mainly supportive, however, riluzole is a glutamate-blocker agent that has been shown to delay death in amyotrophic lateral sclerosis.
Polio
Poliomyelitis, or polio, is a viral disease that causes inflammation of the grey matter of the spinal cord, notably the ventral horn.
Symptoms of poliomyelitis include muscle weakness and flaccid paralysis.
Syphillis
Tabes dorsalis, also known as syphilitic myelopathy, is a spinal lesion that is the result of tertiary syphilis. It affects the dorsal columns of the spinal cord.
Symptoms of tabes dorsalis include:
Weakness
Paraesthesia(shooting pain)
Impaired proprioception, which manifests as a high-stepping gait
ASA
Anterior spinal artery syndrome is the most common presentation of a spinal artery infarction because of a less efficient blood supply than the posterior spinal arteries.
The ASA is particularly dependent on blood supply from the radicular arteries that originate from the thoracic aorta, such as the artery of Adamkiewicz. Thoracic aortic surgery can result in reduced blood flow through the radicular arteries (eg, from aortic cross-clamping and/or systemic hypotension) and consequently lead to anterior spinal cord infarction.
In anterior spinal artery syndrome, the motor and sensory tracts of the anterior part of the spinal cord are affected, which include:
Corticospinal tract
Spinothalamic tract
Ventral horn
Lateral grey matter
The symptoms of anterior spinal artery syndrome include:
Bilateral loss of pain and temp (one level below lesion)
Bilateral spastic paresis (below lesion). Bilateral flaccid paralysis (level of lesion - lower motor neuron symptom).
The flaccid paralysis is due to spinal shock. Upper motor neuron signs such as spasticity and hyperreflexia subsequently develop over days to weeks.
Symptoms of bowel and bladder dysfunction (eg, urinary retention) can result from autonomic dysfunction due to involvement of the intermediolateral cell column and its descending tracts.
B12 Deficiency
Vitamin B12 deficiency leads to a spinal lesion called subacute combined degeneration, which results in demyelination of neurons in the dorsal columns and corticospinal tract.
The symptoms of vitamin B12 deficiency related spinal lesions are a loss of vibration and discrimination and a spastic paresis affecting the legs first, both occurring bilaterally. Note that B12 deficiency can also lead to ataxia, altered mental status, dementia, personality changes, fatigue, and hyperreflexia.
Subacute combined deficiency can also be caused by vitamin E deficiency. By looking at blood work, you can determine the cause of subacute combined degeneration - B12 deficiency causes megaloblastic anemia and vitamin E deficiency causes hemolytic anemia.
Syringomyelia
Syringomyelia is the development of a centralized cavity (syrinx) within the spinal cord, which expands and compresses adjacent neural tissue.
The spinal tracts affected in syringomyelia are the ventral horn and ventral white commissure.
The symptoms of syringomyelia include bilateral loss of pain and temperature one level below the lesion and bilateral flaccid paralysis at the level of the lesion.
Pathogenesis
The exact pathophysiology of syringomyelia is unknown, but involves post-traumatic cystic degeneration of the spinal cord.
Central cord syndrome (CCS) typically occurs with hyperextension injuries in elderly patients with pre-existing degenerative changes in the cervical spine.
Syringomyelia is most common amongst men between the third and fourth decades of life.
In the early stage, syringomyelia first presents with loss of pain and temperature sensation due to pressure on the anterior white commissure, which affects the spinothalamic tracts. The initial presentation is commonly referred to as a cape like distribution of loss, first affecting the back and arms.
As the cavity within the spinal canal continues to expand and affect the ventral horns, neurological symptoms include:
Flaccid paralysis
Decreased deep tendon reflexes
Fasciculations
CCS is characterized by weakness that is more pronounced in the upper extremities than the lower.
MRI is used in syringomyelia to visualize expansion of the syrinx.
Treatment of syringomyelia involves surgical decompression, with shunting indicated in recurrent cases.
Brown-Sequard
Brown-Sequard is a spinal lesion that is the result of a lateral hemisection of the spinal cord, which affects all tracts on that side of the spinal cord.
Because all spinal tracts are affected in Brown-Sequard syndrome, the symptoms include:
Ipsilateral loss of vibration and discrimination below the lesion
Ipsilateral spastic paresis below the lesion
Ipsilateral flaccid paralysis at the level of the lesion
Contralateral loss of pain and temperature two levels below the lesion (the spinothalamic tract ascends contralaterally in the spinal cord)
Alcoholic Cerebellar Degeneration
cause: >10 years heavy alcohol use. Degeneration of Purkinje cells (cerebellar vermis) responsible for truncal coordination
symptoms:
Usually develops over weeks to months
Wide-based gait
Incoordination in legs
Cognition usually intact
PE:
Impaired tandem walking/heel-knee-shin
Preserved finger-nose testing
diagnosis:
CT/MRI - cerebellar atrophy
treatment: alcohol cessation, nutrient supplementation
Last updated